Preparation process and application of interleukin-10
A technology for the preparation of interleukins, which is applied to medical preparations containing active ingredients, peptide/protein components, and the use of carriers to introduce foreign genetic materials. The effect of reducing the loss of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
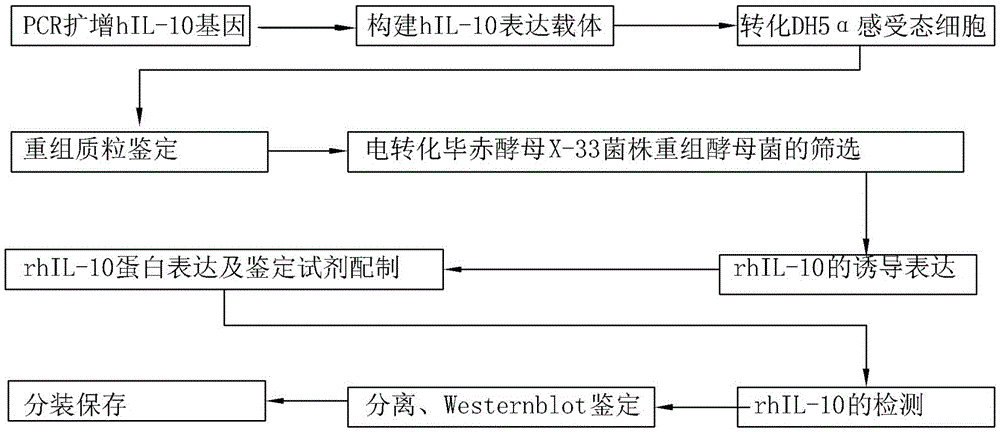
[0055] Such as figure 1 Shown: the preparation process of interleukin-10, including preparation by the following steps:
[0056] Step 1, PCR amplification of the hIL-10 gene: according to the full length of the hIL-10 gene and the multiple cloning site in the plasmid pPICZαA, primers were designed with the software PimerPremier.
[0057] Upstream primer: 5'CG GAATTC ATGGCCCACAGCTCAGCA3',
[0058] Downstream primer: 5'GC TCTAGA CAGTTTCGTATCTTCATTGTC3'.
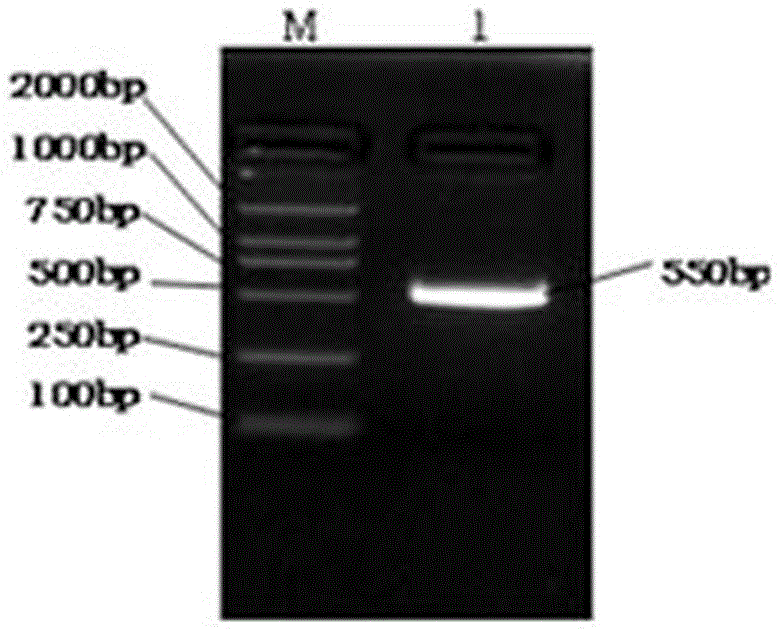
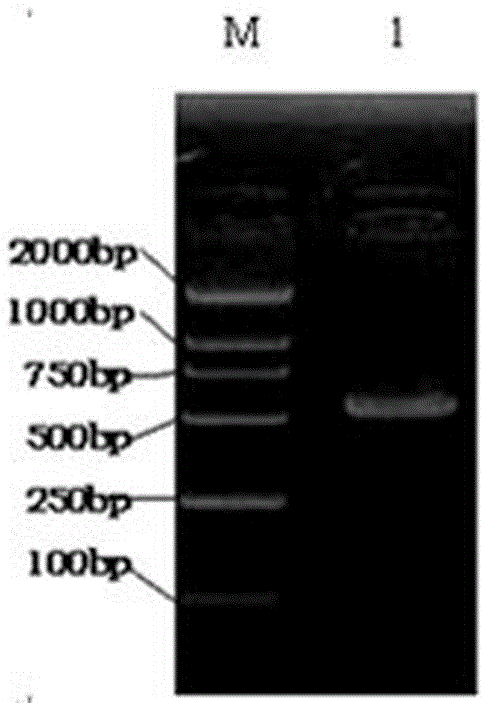
[0059] The part underlined in italics is the introduced EcoRI site XbaI site. Using the plasmid pORF-hIL-10 as a template, hIL-10 was amplified by PCR. The PCR reaction conditions were: in a 50μl reaction system, 94°C, pre-denatured for 10min, 94°C for 45s, 52°C, annealed for 45s, 72°C, extended for 1min , 35 cycles, 72°C extension 10min. After PCR, the amplification results were observed by 1.0% agarose gel electrophoresis as follows: figure 2 As shown in the electrophoresis results, it can be seen that the PCR a...
Embodiment 2
[0170] Example 2: Application in Anti-Skin Graft Rejection in Rabbits
[0171] 1. Application of rhIL-10 anti-rabbit skin graft:
[0172] (1) Optimizing the conditions of the rabbit skin transplantation model, three recipient areas were prepared on the right side of the back of each rabbit, and the skin was peeled, with an area of 1.5×1.5cm 2 , with neat edges. When taking the skin, it is advisable that there are many dense and small bleeding spots on the wound. The optimal thickness of the skin graft is 10-15 mm, including the dermis, and the basement membrane is removed, and the skin graft is trimmed; the operation time is closely related to the survival time, and the operation time within 30 minutes is conducive to the survival of the skin graft.
[0173] (2) Rabbit skin transplantation experiments were administered from the day before transplantation for 10 consecutive days.
[0174] (3) Dosage and method: the first group rhIL-10 dose was 10 μg / kg / d, intramuscular inj...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com