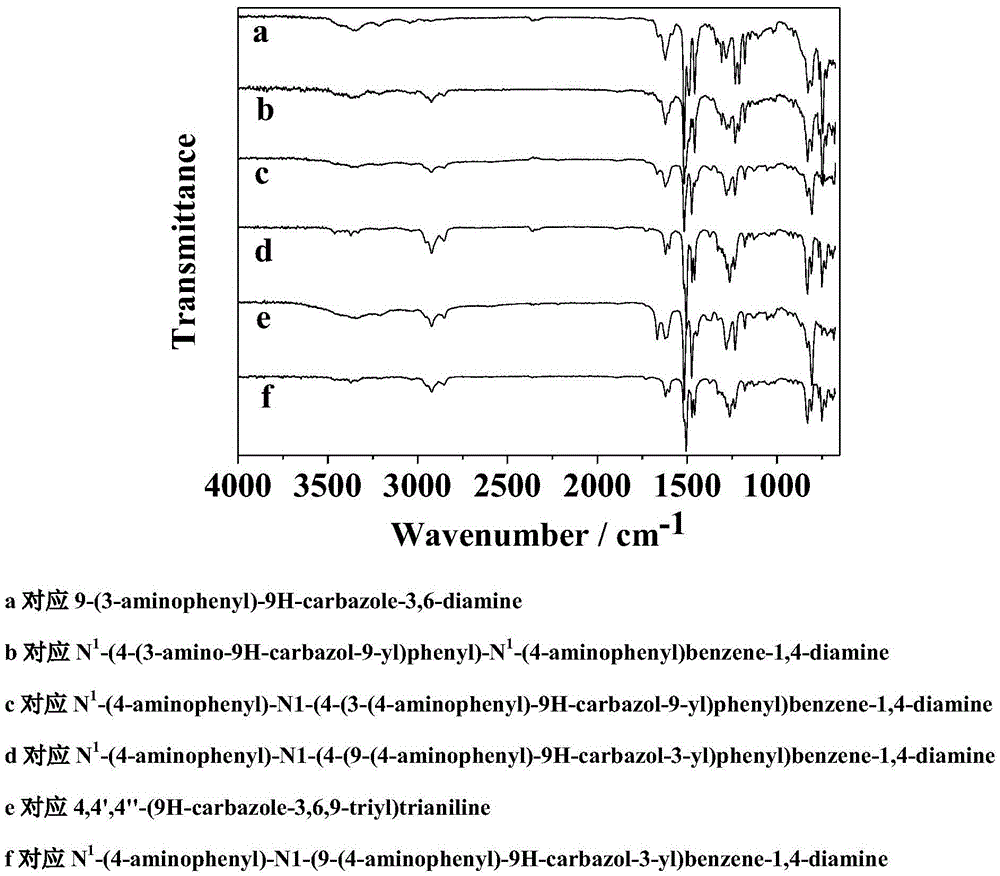
Novel functional triamine monomer containing carbazole structure and preparation method and application thereof
A technology of triamine monomers and monomers, applied in the field of material science, to achieve the effects of high yield, easy purification, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 9-(3-aminophenyl)-9H-carbazole-3,6-diamine:
[0031]
[0032] (1) Synthesis of intermediate 3,6-dinitro-9H-carbazole:
[0033] Combine 4.180g (0.025mol) 9H-carbazole, 7.000g (0.030mol) Cu(NO 3 ) 2 ·2.5H 2 O, 20ml acetic acid, and 30ml acetic anhydride were added to a 500ml three-necked flask and stirred at room temperature for 30min. The temperature was raised to 100°C for 30 minutes. Then add 10ml of acetic acid and 250ml of water, filter the reaction solution with suction, and wash the filter cake five times in 100ml of distilled water; then dissolve the filter cake in a mixed solution of 20gKOH, 250ml of water and 250ml of ethanol, continue to stir for 30min and then pump filter. The filtrate was acidified with concentrated hydrochloric acid and left to stand for 30 min. The yellow precipitate was collected by suction filtration. The product was purified by column chromatography with dichloromethane: n-hexane=1:2 (volume ratio) as the mobile phase and silic...
Embodiment 2
[0041] N 1 -(4-(3-amino-9H-carbazol-9-yl)phenyl)-N 1 -(4-aminophenyl)benzene-1,4-diamine synthesis:
[0042]
[0043] (1) Synthetic intermediate 9-(4-nitrophenyl)-9H-carbazole:
[0044] Add 1.672g (0.01mol) carbazole, 2.122ml (0.02mol) p-fluoronitrobenzene and 50ml DMF into a 100ml three-necked flask, stir magnetically and ventilate with argon, then add 1.122g (0.01mol) potassium tert-butoxide, The temperature was raised to 110°C for 24h. The reaction solution was poured into ice water, the precipitate was filtered out and crystallized twice with ethanol, and the filter cake obtained by suction filtration was dried at 80° C. under vacuum for 24 hours to obtain 2.566 g of the product with a yield of 89%. The intermediate structure is as follows:
[0045]
[0046] (2) Synthesis of intermediate 4-(9H-carbazol-9-yl)aniline:
[0047] Add 2.883g (0.01mol) 9-(4-nitrophenyl)-9H-carbazole into a 500ml three-necked flask, add 300ml absolute ethanol, magnetically stir and argon gas, after heat...
Embodiment 3
[0058] N 1 -(4-aminophenyl)-N 1 Synthesis of -(4-(3-(4-aminophenyl)-9H-carbazol-9-yl)phenyl)benzene-1,4-diamine:
[0059]
[0060] (1) Synthesis of intermediate 3-bromo-9-(4-nitrophenyl)-9H-carbazole:
[0061] Add 2.461g (0.01mol) 3-bromocarbazole, 2.122ml (0.02mol) p-fluoronitrobenzene and 50ml DMF into a 100ml three-necked flask, stir magnetically with argon, and then add 1.122g (0.01mol) tert-butanol Potassium, heated to 110 ℃ for 24h. The reaction solution was poured into ice water, the filter cake obtained by suction filtration was recrystallized twice with ethanol, and the precipitate was filtered out and dried under vacuum at 80° C. for 24 hours to obtain 3.158 g of the product with a yield of 86%. The intermediate structure is as follows:
[0062]
[0063] (2) Synthesis of intermediate 4-(3-bromo-9H-carbazol-9-yl)aniline:
[0064] Add 3.672g (0.01mol) 3-bromo-9-(4-nitrophenyl)-9H-carbazole to a 500ml three-necked flask, add 450ml absolute ethanol, magnetically stir and argon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com