Synthesis method of 4(5)-hydroxymethylimidazole
A technology for the synthesis of hydroxymethylimidazole and its synthesis method, which is applied in the field of synthesis of 4-hydroxymethylimidazole, can solve problems such as excessive generation of industrial waste liquid, unfavorable industrialization promotion, and unsatisfactory yield, and achieve high industrial promotion value, Strong selectivity and effect of increasing concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] Loaded Ni-Cu / SiO 2 The preparation method of catalyst comprises the following steps:
[0064] The copper nitrate of 1.268g and the nickel nitrate of 1.652g were dissolved in 10mL deionized water, and then the solution obtained was added dropwise to 3gSi0 2 and stir rapidly with a glass rod, place the resulting mixture in the air for 12h, put it into a 120°C oven and heat it for 12h, and then roast it at 500°C for 3h to obtain a catalyst precursor. Hydrogen is produced after reduction at 350°C for 1h.
[0065] Add load Ni-Cu / SiO 2 The catalyst can effectively catalyze the reduction of 4(5)-imidazole ethyl carboxylate to obtain 4(5)-hydroxymethylimidazole, with the support of Ni-Cu / SiO 2 With the increase of the amount of catalyst added, the corresponding product yield is also improved. According to the experimental data, the present invention adopts the supported Ni-Cu / SiO 2 The catalyst addition mass is 8-12g, wherein when the addition mass is 10g, the yield is the hi...
Embodiment 1
[0067] A kind of synthetic method of 4(5)-hydroxymethylimidazole, comprises the following operation steps:
[0068] The first step: decarboxylation reaction:
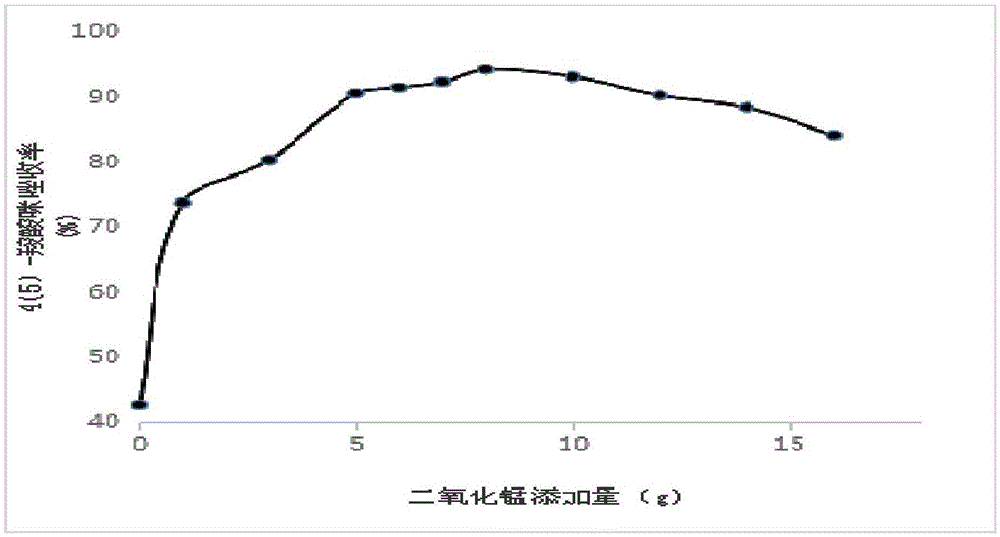
[0069] Mix 40g of 4,5-dicarboxylic imidazole, 1200mL of acetic anhydride, and 5g of manganese dioxide solid under reflux for 8 hours, filter and concentrate the filtrate to dryness.
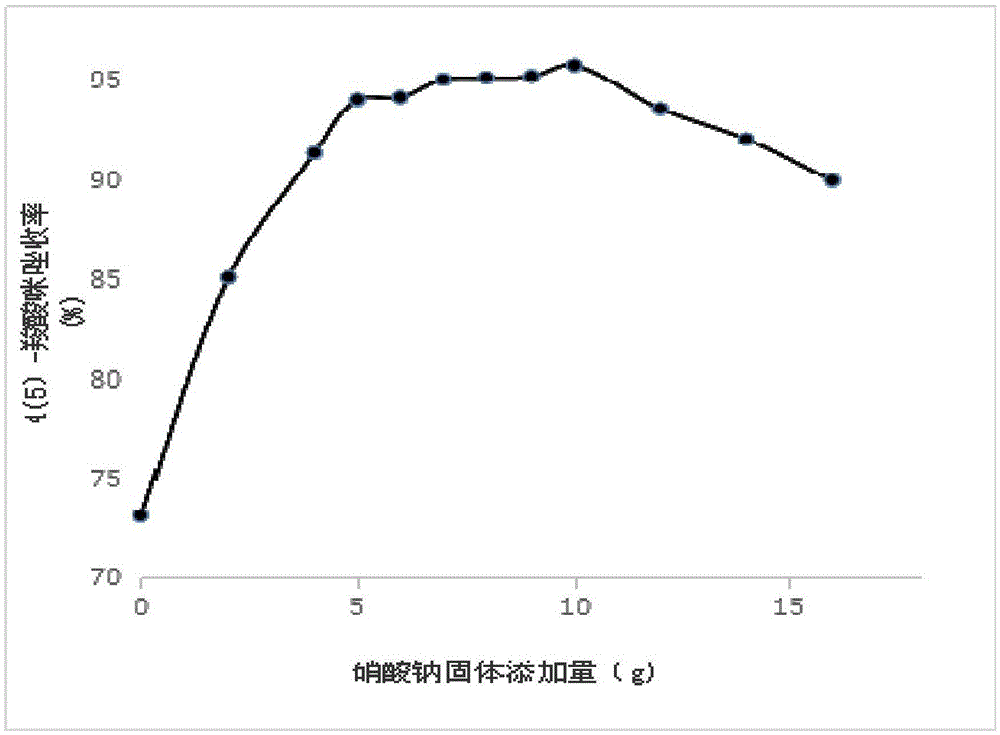
[0070] Add 600 mL of 50% ethanol aqueous solution to the obtained solid and heat to reflux for 2 hours. After heating to reflux, filter while it is hot and add 10 g of solid sodium nitrate to the filtrate, stir well, let it cool naturally overnight, and filter again after overnight to obtain solid 4(5)-carboxylic acid imidazole;
[0071] The second step: esterification reaction:
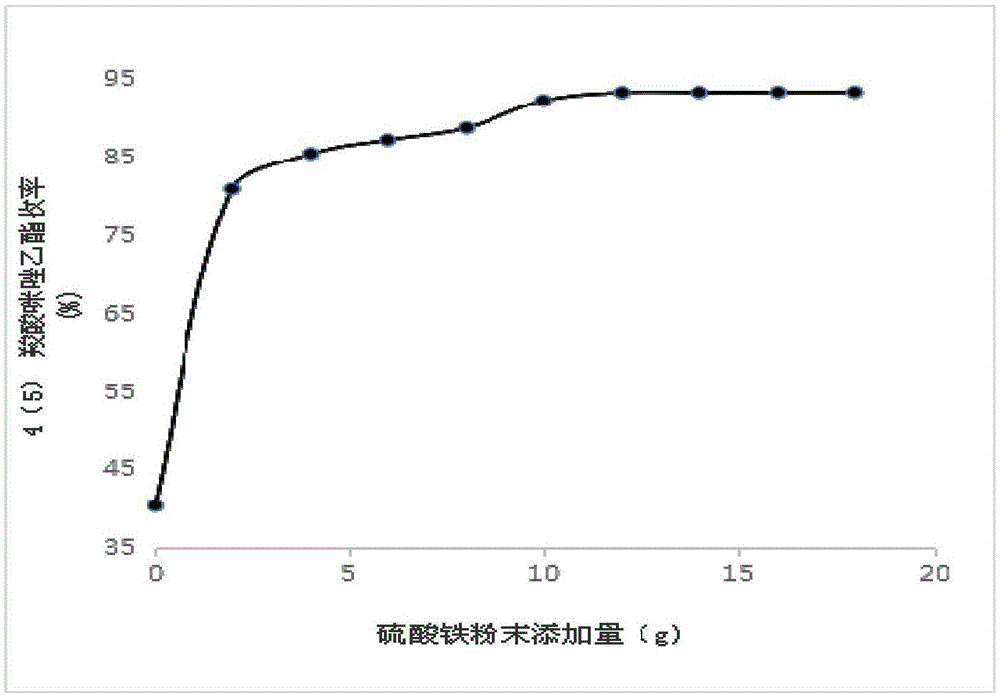
[0072] Mix 50g of 4(5)-imidazole carboxylate, 1000mL of ethanol, 30mL of concentrated sulfuric acid and 10g of ferric sulfate powder and heat to reflux for 2h. After the reflux reaction, cool to room temperature and add 5% NaOH aqueous solution dropwise to adju...
Embodiment 2
[0076] A kind of synthetic method of 4(5)-hydroxymethylimidazole, comprises the following operation steps:
[0077] The first step: decarboxylation reaction:
[0078] Mix 40g of 4,5-dicarboxylic imidazole, 1200mL of acetic anhydride, and 6g of manganese dioxide solids, heat to reflux for 8h, filter and concentrate the filtrate to dryness,
[0079] Add 600 mL of 50% ethanol aqueous solution to the obtained solid and heat to reflux for 2 hours. After heating to reflux, filter while it is hot and add 8 g of solid sodium nitrate to the filtrate, stir evenly, let it cool naturally overnight, and filter again after overnight to obtain solid 4(5)-carboxylic acid imidazole;
[0080] The second step: esterification reaction:
[0081] 50g of 4(5)-carboxylic acid imidazole, 1000mL of ethanol, 30mL of concentrated sulfuric acid and 10.5g of iron sulfate powder were mixed and heated to reflux for 2h. Concentrate under pressure to dryness to obtain mixed solids, add 30mL of water to refl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com