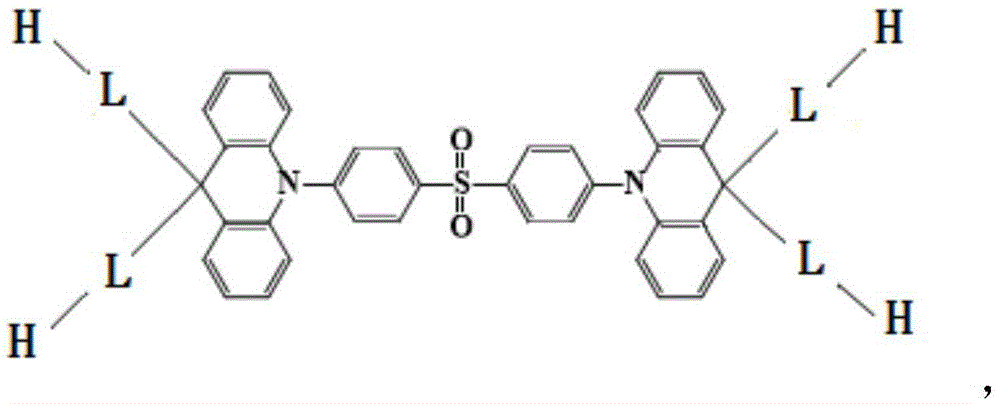
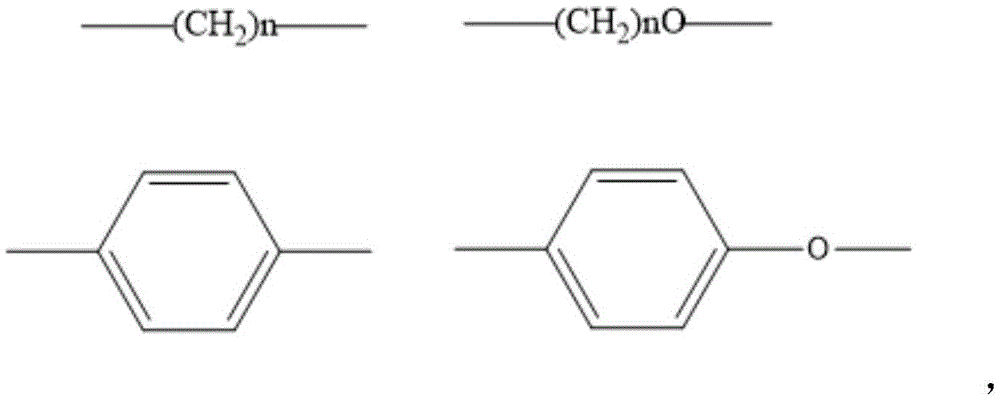
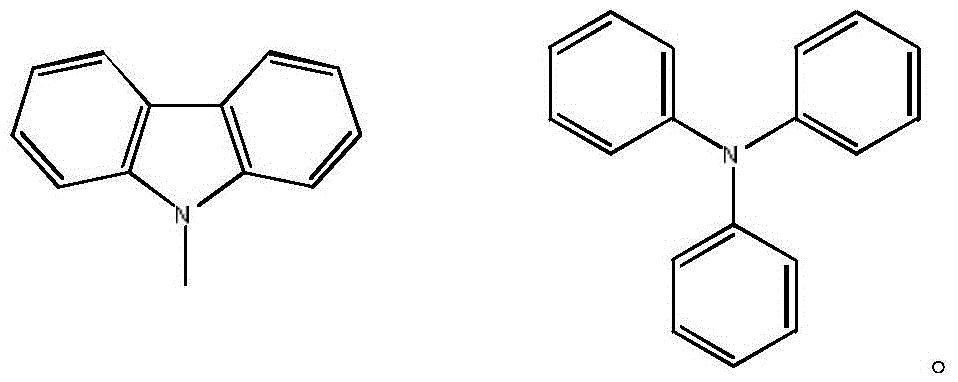
Dendritic thermal activation delay fluorescence material and synthesizing method thereof
A thermal activation delay, fluorescent material technology, applied in the direction of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of easy phase separation, difficult material purification, low efficiency roll-off, etc., to achieve easy separation and purification, The preparation method is simple and effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Synthesis of 1-(benzyloxy)-4-bromobenzene
[0031] Weigh sodium hydride (2.4g, 60mmol, 60%), 70mL of dry DMF in a 250mL three-necked flask, change nitrogen for three times, and cool to 5°C in an ice water bath. P-bromophenol (8.651g, 50mmol) was quickly added to the system, and the reaction was stirred for 30 minutes until no bubbles emerged. Benzyl bromide (8.551 g, 50 mmol) was injected into a constant pressure funnel and slowly dropped into the system. After dripping, the ice-water bath was removed, and it was naturally raised to room temperature to react and stir for 2 hours. After the reaction, it was poured into ice-saturated ammonium chloride solution for quenching, stirred for half an hour, and a large amount of white solid was precipitated. Filter with sand core funnel and wash with ice methanol. The filter cake was recrystallized with ethanol to obtain 11.678 g of pure white fine needle-like solid, melting point: 62-64°C, yield: 89%.
[0032] (2) Synthesis ...
Embodiment 2
[0054] (1) Synthesis of 1-[4-(N-(Diphenylamino)phenyl)methoxy]-6-bromohexane
[0055] The synthetic route is shown in the following formula:
[0056]
[0057] The specific synthesis steps are as follows:
[0058] Weigh diphenylamino-4-benzyl alcohol (5.5g, 20mmol) and sodium hydride (2.4g, 60mmol, 60%) in a 250mL three-neck flask, change nitrogen for three times, inject 50mL tetrahydrofuran, and stir at room temperature to react for 1 hour until no bubbles Pop up. Weigh 1,6-dibromohexane (19.517g, 80mmol) in another 250mL single-neck flask, change nitrogen for three times, inject 50mL dry tetrahydrofuran, and heat to 65°C. Pour the prepared sodium alkoxide into the constant pressure funnel and add slowly. After the addition, react at 65°C overnight. After the reaction is over, cool to room temperature, pour into ice ammonium chloride solution for quenching, extract with ethyl acetate, combine the organic layers, wash with saturated sodium chloride solution, and dry with anhydrous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com