Preparation and application of mercury ion fluorescent probe compound based on rhodamine B
A fluorescent probe and compound technology, applied in the field of fluorescent probes, can solve the problems that the probe does not provide the lowest detection limit, cannot quantitatively measure the content of mercury ions, and is difficult to meet low-concentration tests, so as to achieve good sensitivity and selectivity , good promotion prospects, good water-soluble effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
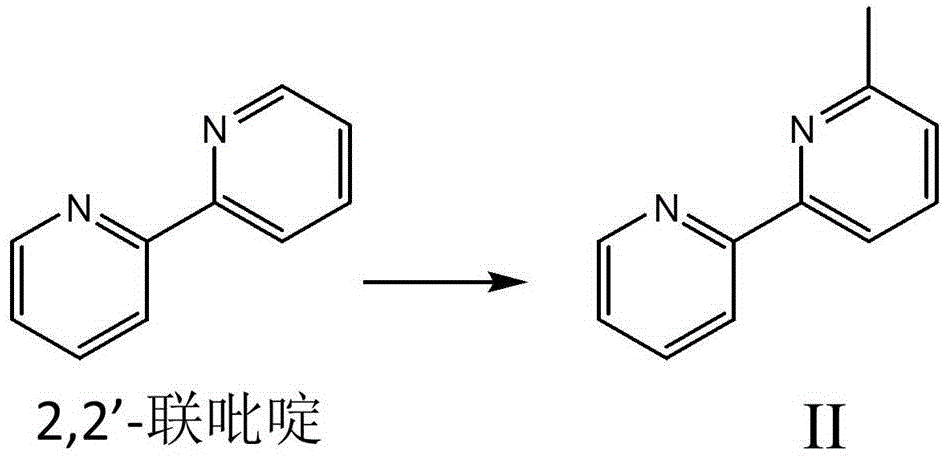
[0043] The synthesis of embodiment 1,6-methyl-2,2'-bipyridine ( figure 2 )
[0044]Take 5.30 g of 2,2'-bipyridine and place it in a three-necked flask, protect it with nitrogen and use an ice bath to lower it below 0°C, then take 100 ml of dry ether and add it to the flask until the 2,2'-bipyridine is completely dissolved; 26 milliliters (1.3 moles per liter) of methyllithium was added dropwise into the flask, reacted under ice cooling for 2 hours, and then heated to reflux for 3 hours. After cooling to room temperature, 10 ml of water was added to quench the reaction. After liquid separation, the aqueous phase was extracted with ether, the solvent was evaporated, and 300 ml of saturated potassium permanganate in acetone was added, stirred for one hour, and the manganese dioxide generated was removed by filtration. The solvent was distilled off, and 3.728 g of pure product II was distilled under reduced pressure, with a yield of 63.8%. H NMR spectrum: 1 HNMR (400MHz, CDCl ...
Embodiment 2
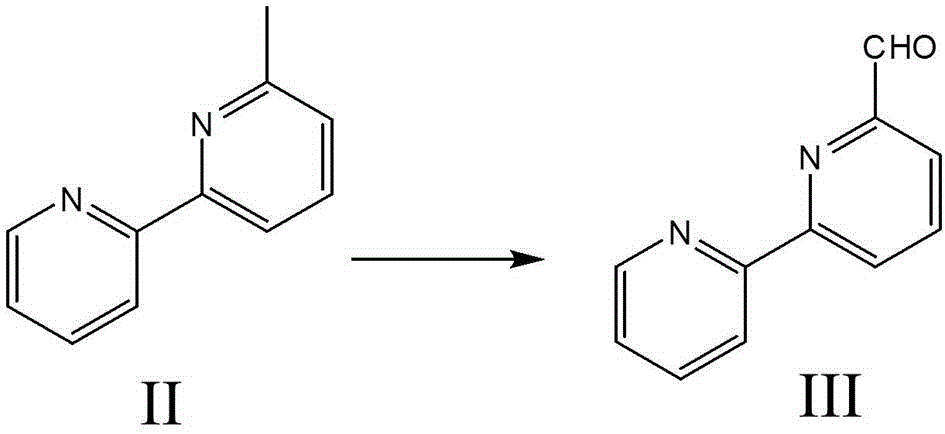
[0045] Embodiment 2, the synthesis of 6-formyl-2,2'-bipyridine ( image 3 )
[0046] Take 3.31 grams of 6-methyl-2,2'-bipyridine, 3.24 grams of selenium dioxide, and 0.21 milliliters of water, mix and dissolve in 50 milliliters of dioxane, heat and reflux for 3 hours, add 3.24 grams of selenium dioxide and 0.21 ml of water was heated to reflux for 27 hours. After cooling to room temperature, the solvent was distilled off, and dichloromethane:methanol=15:1 was used as a developing solvent, and the column was separated to obtain 1.28 g of pure product III with a yield of 31%. H NMR spectrum: 1 HNMR (400MHz, CDCl 3 )δppm: 10.18(s,1H),8.74(d,J=4.83Hz,1H),8.65(dd,J=7.12,1.95Hz,1H),8.56(d,J=7.95Hz,1H),7.97- 8.02 (m, 2H), 7.90 (td, J=7.65, 1.64Hz, 1H), 7.40 (ddd, J=7.57, 4.87, 1.10Hz, 1H).
Embodiment 3
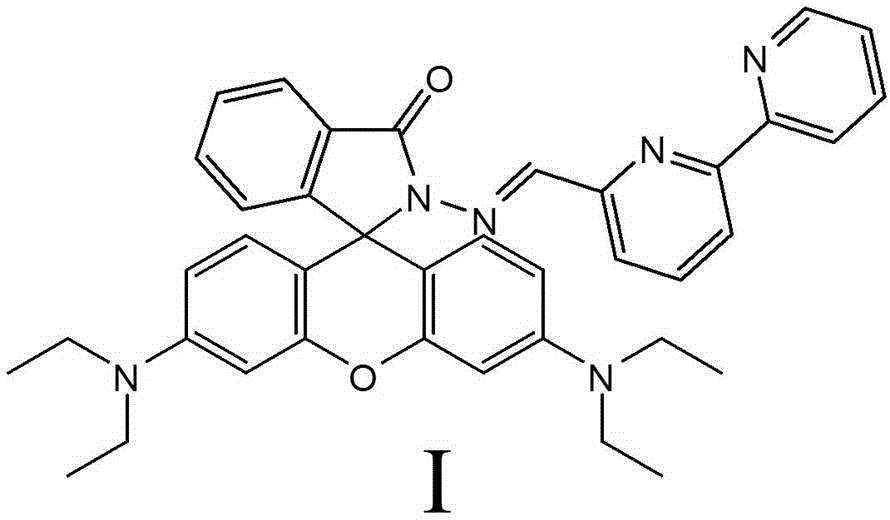
[0047] Embodiment 3, the synthesis of fluorescent probe of the present invention ( Figure 4 )
[0048] Take 0.18 g of 6-formyl-2,2'-bipyridine and 0.55 g of rhodamine B hydrazide, dissolve it in 20 ml of ethanol, and heat to reflux for 6 hours. The product was separated with a basic silica gel column, and dichloromethane was used as a developing solvent to obtain 0.362 g of pure product probe with a yield of 50%. H NMR spectrum: 1 HNMR (400MHz, CDCl 3 )δppm:8.84(s,1H),8.38(d,J=7.81Hz,2H),7.70-8.03(m,3H),7.3-7.6(m,4H),7.15(d,J=8.27Hz,1H ),6.55(t,J=8.84Hz,1H),6.46(d,J=2.12Hz,1H),6.42(dd,J=7.81,2.69Hz,2H),6.25(m,2H),3.33(dt ,J=13.93,7.17,8H), 1.15(dd,J=12.72,7.02Hz,12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com