Coumarin schiff-base copper ion complex-based fluorescent probe for thiol as well as preparation method and application thereof
A technology of Schiff base copper and fluorescent probes, applied in the field of applied probes, can solve the problems of long incubation time, low practicability, minutes to 1 hour or even longer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Reagent
[0034] 4-Diethylamino salicylaldehyde, diethyl malonate, piperidine, 80% hydrazine hydrate, 2,6-pyridinedicarbaldehyde, perchlorates of various metal ions (Cr 3+ , Ag + , Fe 3+ ,K + , Na + ,Mg 2+ ,Pb 2+ , Ca 2+ ,Hg 2+ ,Mn 2+ ,Cd 2+ , Fe 2+ ,Zn 2+ , Ni 2+ ,Co 2+ ,Cu 2+ ) in aqueous solution (2.0×10 -2 M), the aqueous solution of various amino acids (2.0×10 -2 M), absolute ethanol, acetonitrile (chromatographically pure), dimethylformamide (analytical pure), HEPES (pH: 7.35-7.45) solution, etc.
[0035] Synthesis
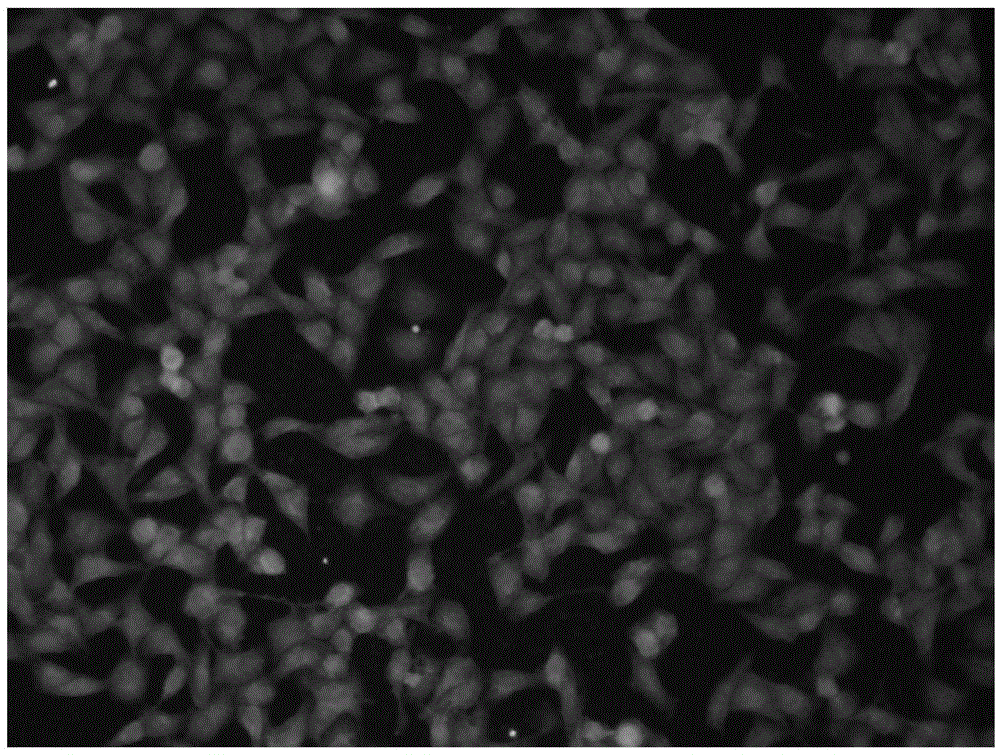
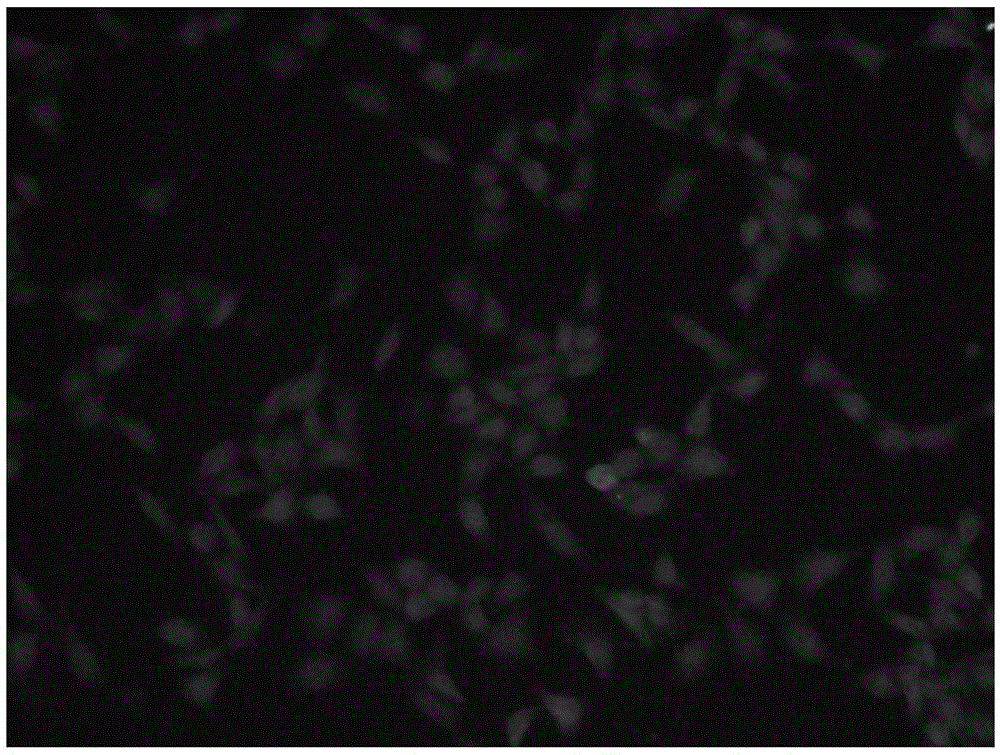
[0036] The synthesis process shown in formula 1: 7-N,N-dimethylamino-2-oxo-2H-3-coumarate (compound 3) and compound 2 were synthesized by prior art. Compound 1 is synthesized by compound 2 and 2,6-pyridinedicarbaldehyde, and is characterized by hydrogen spectrum, carbon spectrum and mass spectrum ( Figure 6 ,7,8). Compound 1-Cu 2+ is composed of compound 1 and Cu(ClO 4 ) 2·6H 2 O prepared.
[0037]
[0038] Compound 1 vs. ...
Embodiment 2
[0048] Synthesis
[0049] Synthesis of Compound 3 (7-N,N-Dimethylamino-2-oxo-2H-3-coumarate)
[0050] 4-Diethylaminosalicylaldehyde (7.72 g, 0.04 mol), diethyl malonate (12.8 g, 12.16 mL, 0.08 mol) and piperidine (4 mL) were mixed in absolute ethanol (120 mL). The mixture was refluxed for 6 hours with stirring. After cooling to room temperature, rotary evaporation until the ethanol solvent was no longer evaporated, a small amount of oily liquid was obtained which was compound 3.
[0051] Synthesis of compound 2 (coumarin hydrazide)
[0052] Weigh 4.3400 g of compound 3 (7-N, N-dimethylamino-2-oxo-2H-3-coumarate, 15 mmol) and dissolve it in 40 ml of ethanol, add 3.6381 ml of 80% hydrazine hydrate ( Density = 1.032 g / ml, 60 mmol). After stirring at room temperature for 12 minutes, it was cooled in ice water for 15 minutes. The resulting precipitate was filtered with suction to obtain the product compound 2 (coumarin hydrazide).
[0053] Synthesis of compound 1
[0054] 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com