Method for detecting human leukocyte antigen HLA-G
A technology for HLA-G and leukocyte antigen, applied in the field of detection of serum soluble human leukocyte antigen G, can solve the problems of unsatisfactory sensitivity and accuracy, achieve good repeatability and accuracy, high detection sensitivity, and good specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Implementation 1. Recombinant HLA-G protein fragments
[0040] Materials and methods
[0041] 1. Cloning of target gene and construction of plasmid
[0042] ●Get the HLA-G gene sequence from the gene bank, select the regions with low homology with HLA-A, -B and -C, and design primers with Primer5.0 software. The upstream primer is 5'-gaagaggagacacggaacac-3'(255---274bp) and the downstream primer is 5'-ctccaggtaggctctccttt-3'(538---557bp). And the designed primers were handed over to Sangon Biological Engineering (Shanghai) Co., Ltd. for synthesis.
[0043] ●Because the human placental tissue has a high expression of HLA-G, the placental tissue was homogenized, and the total RNA was extracted by TRNzol method.
[0044] ●Two-step method for RT-PCR to amplify the target gene to be cloned. The reverse transcription (RT) reaction system and reaction conditions are shown in Table 1; the polymerase chain reaction (PCR) reaction system and reaction conditions are shown in T...
Embodiment 2
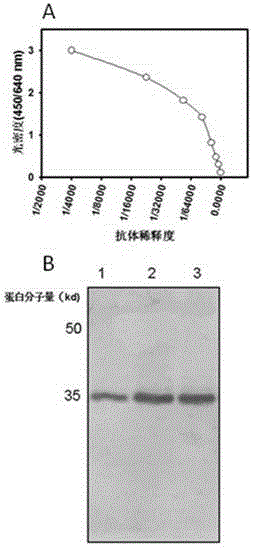
[0064] Implementation 2. Preparation of anti-HLA-G monoclonal antibody
[0065] Materials and methods
[0066] 1. Antigen Preparation
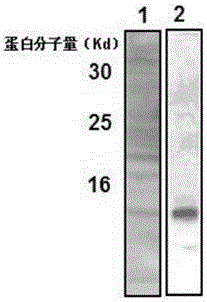
[0067] The HLA-G85aa-185aa fragment prepared in Example 1 was used as an antigen.
[0068] 2. Immunization of mice
[0069] ● After emulsifying 50 μg of HLA-G85aa-185aa fragments with an equal volume of Freund’s complete adjuvant until dripping water does not dissolve, intraperitoneally inject 6-8 week-old female Balb / c mice, the injection volume is 200 μl / mouse, 2 weeks later Perform a second immunization.
[0070] ● After emulsifying HLA-G85aa-185aa fragments with an equal volume of Freund's incomplete adjuvant until dripping does not dissolve, intraperitoneally inject mice with a volume of 200 μl / mouse, and perform the third immunization two weeks later.
[0071] ●After emulsifying the HLA-G85aa-185aa fragment with an equal volume of Freund's incomplete adjuvant until dripping water does not dissolve, inject into the mouse intraperitone...
Embodiment 3
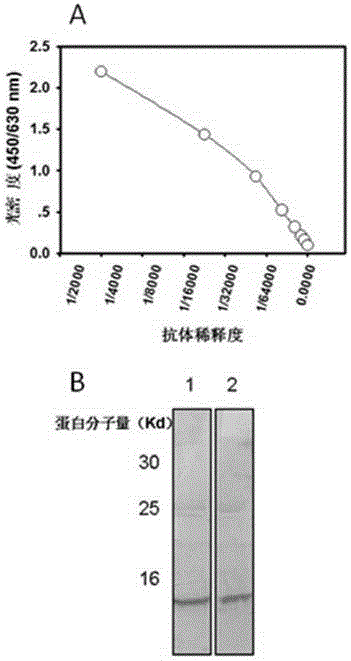
[0088] Implementation 3. Preparation of anti-Beta-microglobulin monoclonal antibody
[0089] Materials and methods
[0090] 1. Antigen Preparation
[0091] Check the information and design the peptide sequence based on β2 microglobulin:
[0092] MSRSVALAVLALLSLSGLEAIQRTPKIQVYSRHPAENGKSNFLNCYVSGF (β2 microglobulin amino acid sequence: 1aa-50aa). The peptide was synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. and coupled to KLH.
[0093] 2. Immunization of mice
[0094] ●After emulsifying 50 μg of KLH-coupled β2 microglobulin polypeptide with an equal volume of Freund’s complete adjuvant until dripping does not dissolve, intraperitoneally inject 6-8 week-old female Balb / c mice, the injection volume is 200 μl / mouse, 2 A second immunization was performed a week later.
[0095] ●After emulsifying 50 μg of KLH-coupled β2 microglobulin polypeptide with an equal volume of Freund’s incomplete adjuvant until it does not dissolve in water, intraperitoneally inject mice wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com