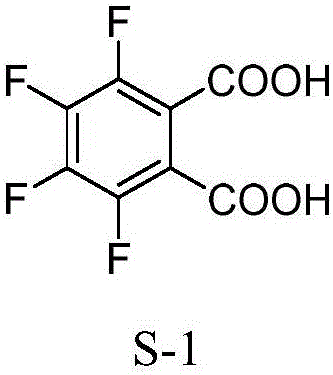
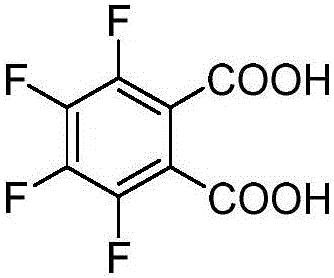
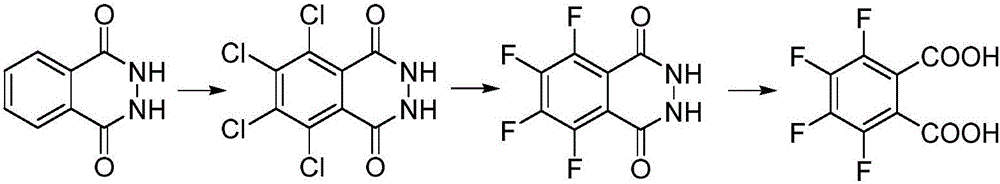
Synthesis method of 3, 4, 5, 6-tetrafluorophthalic acid
A technology of tetrafluorophthalic acid and tetrachlorophthalic hydrazide is applied in the field of synthesis of organic compound 3,4,5,6-tetrafluorophthalic acid, and can solve the problem of unfriendly environment and by-products. many problems, long reaction time, etc., to achieve the effects of broad production prospects, improved product yield, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, a kind of synthetic method of 3,4,5,6-tetrafluorophthalic acid, carries out following steps successively:
[0042] 1), add 16.2g (0.1mol) phthalohydrazide to 50mL oleum (SO 3 The mass fraction is 20%) and 50mL dilute hydrochloric acid (20wt%) in the mixed solution that forms, then add 0.16gKI catalyst and mix and stir, temperature control 50 ℃, control feed 0.3mol chlorine, reaction time 10 hours. The reaction solution after the complete chlorination was cooled (cooled to room temperature) to crystallize, filtered and dried (dried at 80° C. for 60 minutes) to obtain 28.3 g of white crystals of tetrachlorophthalic hydrazide, and the yield was 94.3% (based on o-phenyl Diformyl hydrazide), the content is 99.1%.
[0043] 2) Add 14.53g (0.25mol) of industrial potassium fluoride into a reaction flask containing 50mL of methanol, 50mL of toluene and 50mL of N,N-dimethylformamide mixed solution. The reaction device is equipped with a reflux tube, a thermometer an...
Embodiment 2
[0045] Embodiment 2, a kind of synthetic method of 3,4,5,6-tetrafluorophthalic acid, carries out following steps successively:
[0046] 1), add 16.2g (0.1mol) phthalohydrazide to 50mL fuming sulfuric acid (SO 3The mass fraction is 20%) and 100mL dilute hydrochloric acid (25wt%) mixed solution, then add 0.08gNaI catalyst and mix and stir, the temperature is controlled at 0°C, and 0.4mol chlorine gas is controlled to feed in, and the reaction time is 8 hours. The reaction solution after complete chlorination was cooled and crystallized, filtered and dried to obtain 27.8 g of white crystals of tetrachlorophthalic hydrazide, the yield was 92.7% (calculated as phthalic hydrazide), and the content was 99.0%.
[0047] 2) Add 29.1g (0.5mol) of industrial potassium fluoride into a reaction flask containing 50mL of methanol, 100mL of toluene and 50mL of N,N-dimethylacetamide mixed solution. The reaction device is equipped with a reflux tube, a thermometer and nitrogen replacement devic...
Embodiment 3
[0049] Embodiment 3, a kind of synthetic method of 3,4,5,6-tetrafluorophthalic acid, carries out following steps successively:
[0050] 1) Add 32.4g (0.2mol) phthalohydrazide to 50mL fuming sulfuric acid (SO 3 Mass fraction is 20%) and 150mL dilute hydrochloric acid (28wt%) in the mixed liquor, then add 0.08gI 2 The catalyst was mixed and stirred, the temperature was controlled at 0° C., 1.6 mol of chlorine gas was controlled to flow in, and the reaction time was 8 hours. The reaction liquid after complete chlorination was cooled and crystallized, filtered and dried to obtain 53.1 g of white crystals of tetrachlorophthalhydrazide, the yield was 88.6% (calculated as phthalhydrazide), and the content was 98.8%.
[0051] 2) Add 58.1g (1mol) of industrial potassium fluoride into a reaction flask filled with a mixed solution of 50mL of methanol, 50mL of toluene and 100mL of dimethyl sulfoxide. The reaction device is equipped with a reflux tube, a thermometer and a nitrogen replace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com