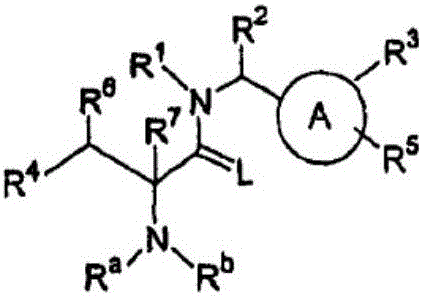
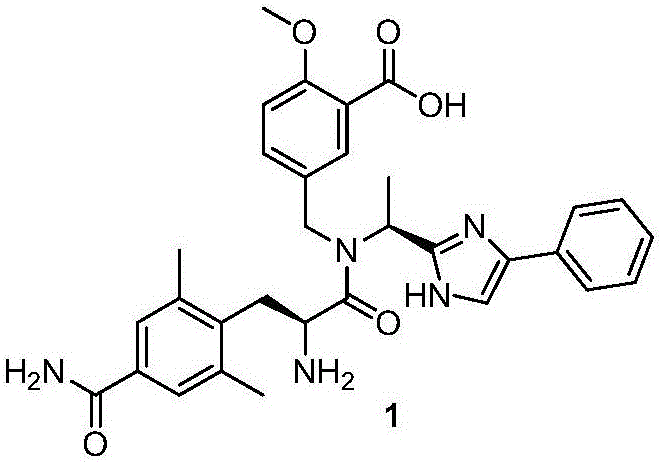
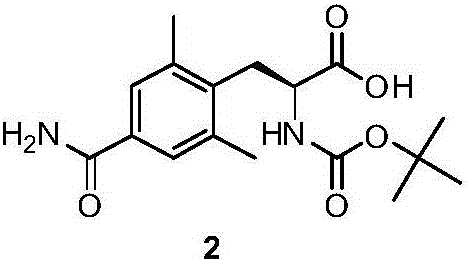
Preparation method of alanine derivatives
A compound and chiral compound technology, applied in the field of preparation of alanine derivatives, can solve the problems of difficult realization, harsh production conditions, and difficult operation, and achieve the goal of reducing production difficulty, mild reaction conditions, and reducing production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The preparation of embodiment 1L-tyrosine methyl ester hydrochloride (compound 11)
[0074]
[0075] Add L-tyrosine (110 g, 0.607 mol) into 500 mL of methanol, cool to 0° C., and add thionyl chloride (108.3 g, 0.91 mol) dropwise. After dropping, the temperature was raised to room temperature naturally, and then heated to reflux for 5 hours, and the reaction was monitored by TLC to complete. Cool to room temperature, filter, wash the filter cake with 420 mL of ethyl acetate, and dry to obtain 140.2 g of white solid, with a yield of 99.7%.
Embodiment 2
[0076] The preparation of embodiment 2N-tert-butoxycarbonyl-L-tyrosine methyl ester (compound 10)
[0077]
[0078] Potassium carbonate (250.9g, 1.815mol) was added to 1.0L of water, stirred evenly, compound 11 (140.2g, 0.605mol) was added under an ice-water bath, and then Boc 2 O (158.4 g, 0.726 mol) in ethanol (300 mL). After dropping, the temperature was naturally raised to room temperature for 2 h, and the reaction was monitored by TLC to complete. Extract with ethyl acetate (600mLX3), combine the organic phases, wash with 1N hydrochloric acid (400mL), tap water (400mL), saturated brine (400mLX2) successively, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain a solid, which was washed with 300 mL of n-hexane and dried to obtain 175.8 g of a white solid with a yield of 98.4%.
Embodiment 3
[0079] Example 3 Preparation of N-tert-butoxycarbonyl-4-trifluoromethanesulfonyloxy-L-phenylalanine methyl ester (compound 9)
[0080]
[0081] (1) Method 1:
[0082] Compound 10 (175.8g, 0.595mol) and 1.2L of dichloromethane were added to the reaction flask, after stirring evenly, pyridine (75.3g, 0.95mol) was added, and then trifluoromethanesulfonic anhydride (201.4g, 0.714mol). Continue to react at 0° C. for 1 h after the drop is complete, and monitor the completion of the reaction by TLC. Add 10% aqueous citric acid (200 mL) to quench the reaction, separate the layers, and wash the organic phase with 10% aqueous citric acid (200 mL) and tap water (200 mL×2), and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure, and then 600 mL of methyl tert-butyl ether was added, frozen to 0°C for crystallization. After filtering, the filter cake was washed with 350 mL of n-hexane, and dried to obtain 239.5 g of lig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com