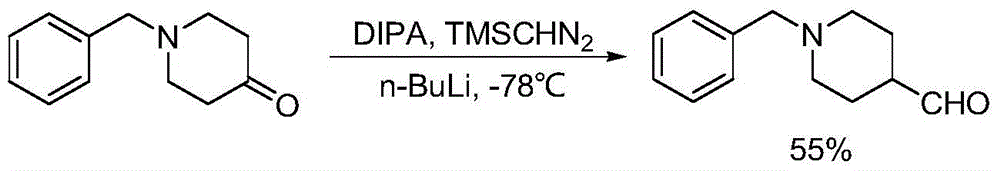
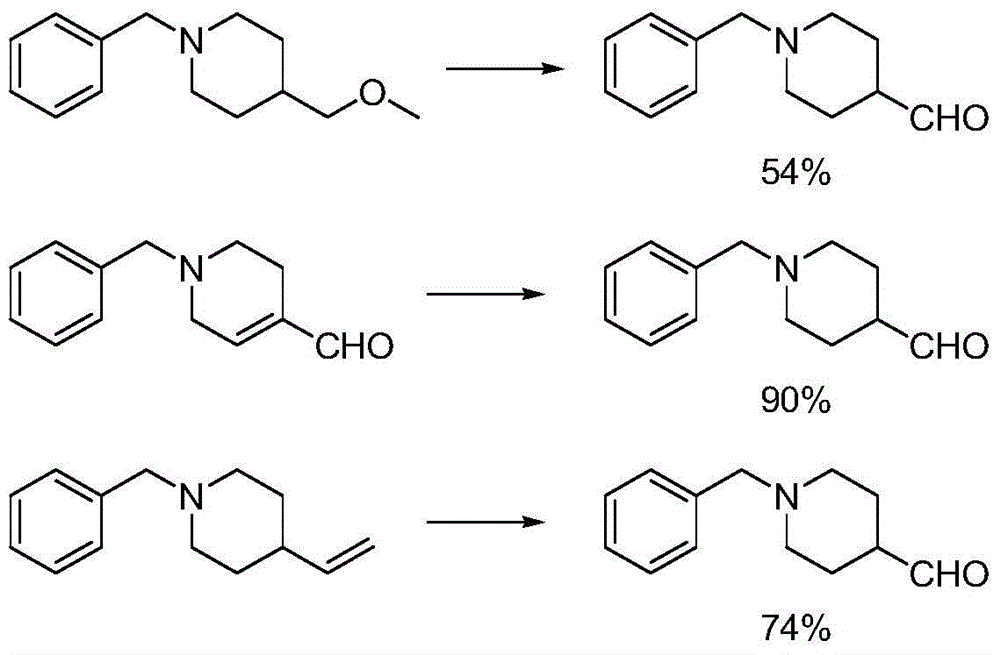
Preparation method of 1-benzyl-4-piperidine formaldehyde
A technology of piperidine formaldehyde and piperidine carboxylic acid, which is applied in the field of preparation of donepezil hydrochloride, can solve the problems of unfriendly environment, harsh reaction conditions, and high production cost, and achieve the effects of good product purity, easy preparation, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 11-benzyl-4-piperidinecarboxylic acid methyl ester
[0041] Add 1.62kg of 4-piperidinecarboxylic acid methyl ester hydrochloride, 1.08kg of benzyl chloride, 1.89kg of sodium bicarbonate and 4.3kg of absolute ethanol into a 20L reaction kettle, heat to reflux, keep the reflux reaction for 3 hours, and cool down to an internal temperature of 30 At about ℃, discharge, filter, wash the filter cake with 2.15kg of absolute ethanol, drain, concentrate the filtrate to obtain a paste, the residue is dispersed in 4.7kg of toluene and 6.0kg of pure water, stir and wash the organic layer, and separate the organic layer, and concentrated toluene to obtain 1.92 kg of methyl 1-benzyl-4-piperidinecarboxylate as a pale yellow oily liquid. Yield: 96.5% (calculated as benzyl chloride). HPLC: 99.12%
[0042] 1 H-NMR (400MHz, CDCl3) δppm: 7.21-7.32 (5H, m), 3.67 (3H, s), 3.48 (2H, s), 2.85 (2H, d), 2.29 (1H, tt), 2.02 (2H ,dt),1.89-1.84(2H,m),1.82-1.71(2H,m...
Embodiment 2
[0044] The preparation of embodiment 21-benzyl-4-piperidinecarboxylic acid ethyl ester
[0045]Add 18.4g of ethyl 4-piperidinecarboxylate hydrochloride, 12.7g of benzyl chloride, 21.0g of sodium bicarbonate and 40ml of absolute ethanol into a 100ml reaction flask, heat to reflux, keep the reflux reaction for 3h, and cool down to an internal temperature of 30°C Left and right, filter, wash the filter cake with 20ml of absolute ethanol, concentrate the filtrate to obtain a paste, add 40ml of toluene and 40ml of water, stir and disperse, separate the organic layer, concentrate toluene to obtain 1-benzyl-4-piperidinecarboxylic acid Ethyl ester 23.2g, is light yellow oily liquid. Yield: 93.8% (calculated as benzyl chloride). HPLC: 98.74%
[0046] 1 H-NMR (400MHz, CDCl3) δppm: 1.24(t, 3H), 1.69-1.82(m, 2H), 1.82-1.92(m, 2H), 1.96-2.10(m, 2H), 2.20-2.34(m, 1H),2.79-2.91(m,2H),3.48(s,2H),4.12(q,2H),7.20-7.32(m,5H)
[0047] ESI(+):248.17
Embodiment 3
[0048] The preparation of embodiment 3 red aluminum-morpholine complexes
[0049] Nitrogen gas was slowly introduced into a 10L dry jacketed reactor, and 2.6kg of toluene and 2.89kg of 70% red aluminum solution were added to the reactor, stirred, and cooled to an internal temperature of -5°C. Dissolve 0.96kg of morpholine in 0.85kg of toluene, drop it into the reactor, and control the reaction temperature to -5°C. After the dropwise addition was completed, the temperature was raised to 15°C, and the stirring reaction was continued for 8h to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com