Preparation method of novel skin filler
A dermal filler, a new type of technology, applied in medical science, prosthesis, etc., can solve the problems of complex post-processing, unsuitable for industrial scale-up, low grafting efficiency, etc., and achieve the effect of prolonging the residual time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
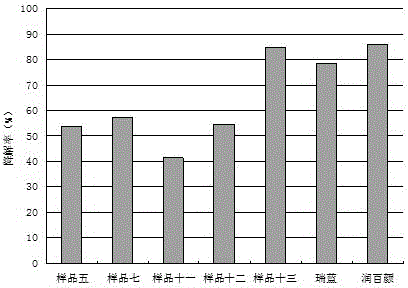
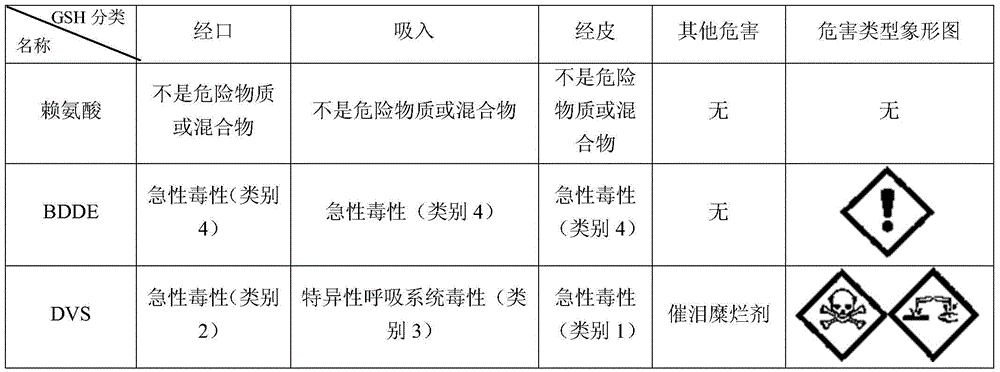
Image
Examples
Embodiment 1
[0018] Dissolve 0.0914g of lysine in 15mL of water, adjust the pH to 4 with hydrochloric acid, weigh 1g of sodium hyaluronate dry powder, stir well, and put it at 4°C overnight. The next day, weigh 0.6918g of DMTMM and dissolve it completely with 5mL of water and add it to the gel. After stirring completely, react at 4°C for 3 days.
[0019] Cut the reacted gel into pieces, dialyze with PBS buffer, change the dialysate once every 1h and weigh it until the gel mass reaches 50g, then stop the dialysis, grind the gel into granules, and obtain cross-linked Sodium hyaluronate gel.
Embodiment 2
[0021] Dissolve 0.0914g of lysine in 15mL of water, adjust the pH to 6 with hydrochloric acid, weigh 1g of sodium hyaluronate dry powder, stir well, and put it at 4°C overnight. The next day, weigh 0.6918g of DMTMM and dissolve it completely with 5mL of water and add it to the gel. After stirring completely, react at 4°C for 3 days.
[0022] Cut the reacted gel into pieces, dialyze with PBS buffer, change the dialysate once every 1h and weigh it until the gel mass reaches 50g, then stop the dialysis, grind the gel into granules, and obtain cross-linked Sodium hyaluronate gel.
Embodiment 3
[0024] Dissolve 0.0914g of lysine in 15mL of water, adjust the pH to 6.5 with hydrochloric acid, weigh 1g of sodium hyaluronate dry powder, stir well, and put it at 4°C overnight. The next day, weigh 0.6918g of DMTMM and dissolve it completely with 5mL of water and add it to the gel. After stirring completely, react at 4°C for 3 days.
[0025] Cut the reacted gel into pieces, dialyze with PBS buffer, change the dialysate once every 1h and weigh it until the gel mass reaches 50g, then stop the dialysis, grind the gel into granules, and obtain cross-linked Sodium hyaluronate gel.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com