Triazole modified mannich base compound and preparation method thereof
A technology of Mannich base and triazole, which is applied in the field of triazole-modified Mannich base compound and its preparation, and oilfield chemicals, can solve the problems of poor corrosion inhibition effect and poor alkali resistance, and achieve alkali resistance Improved performance, improved alkali resistance, and strong acid solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
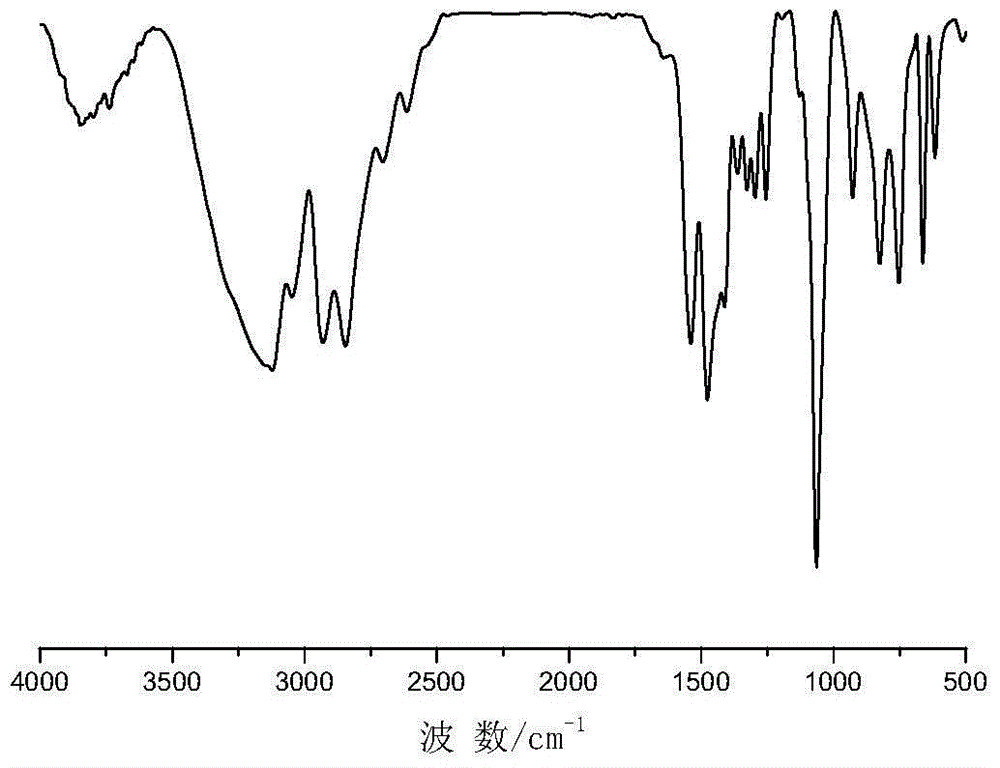
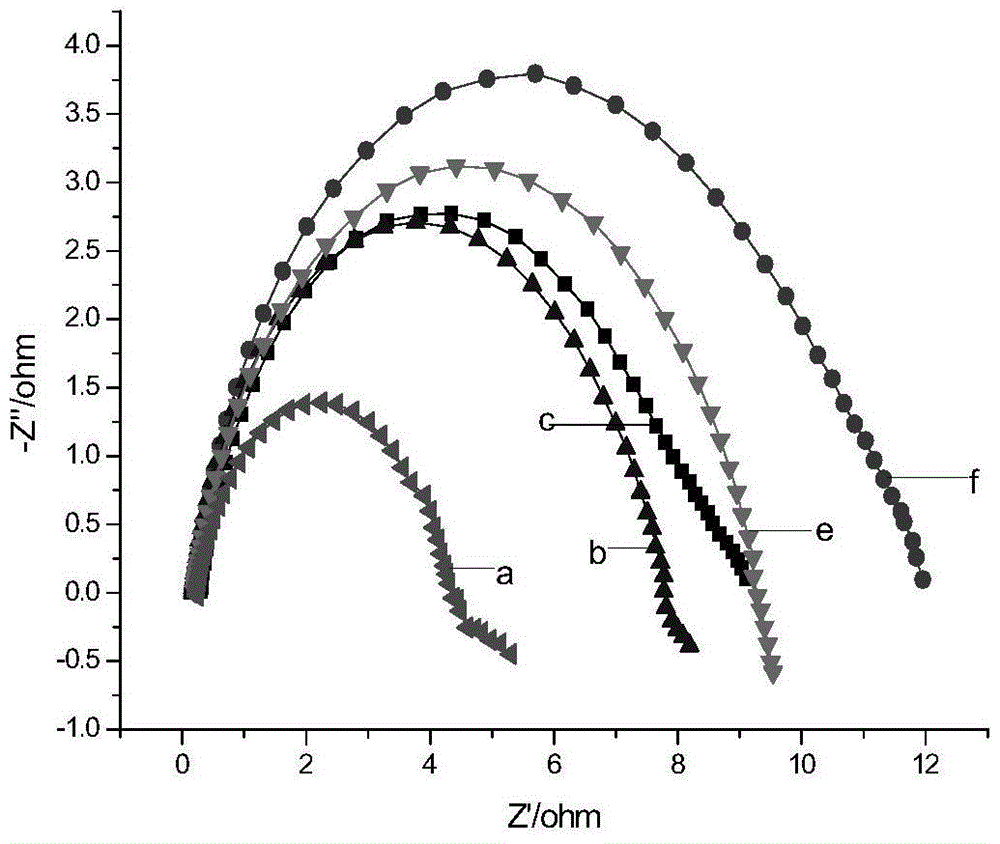
[0033] See Image 6 with Figure 7 , The preparation method of the triazole-modified Mannich base compound of the present invention includes Mannich reaction, preparation of sulfonium ylide, epoxidation and ring opening reaction, and the specific steps are as follows:
[0034] (1): Mix the formaldehyde solution with organic amine (one of diethylamine, diethanolamine, aniline) and add it to the solvent (ethanol or deionized water), and then adjust the pH to 3-6 with hydrochloric acid solution. Under stirring, the temperature is raised to 30~50℃, the reaction is 0.5h~1h, the temperature is continued to 60~90℃, ketone (one of acetone, methyl ethyl ketone, acetophenone) is added dropwise, and the reaction is kept for 5~8h; The solvent was distilled off under reduced pressure, and then washed with ether for 2 to 3 times. After drying, Mannich base was obtained; the mass fraction of the formaldehyde solution was 20%-50%, and the amount of formaldehyde, amines, and ketones in the formald...
Embodiment 1
[0039] The preparation of the triazole modified Mannich base compound includes the following steps:
[0040] (1) Mix 20% formaldehyde solution with diethanolamine and add it to ethanol, then use 15% hydrochloric acid to adjust pH=3.5, stir and raise the temperature to 30°C, react for 1 hour, and continue to heat up to 60°C , Acetone was added dropwise, and the reaction was kept for 8h; the solvent ethanol was removed by vacuum distillation, and then washed with ether for 2 to 3 times, and Mannich base was obtained after drying; among them, the ratio of formaldehyde, diethanolamine, and acetone in the formaldehyde solution It is 1.5:1:1, and the volume ratio of ethanol to formaldehyde solution is 25:1.
[0041] (2) Add CH to a 500mL three-necked flask equipped with an electric stirrer, a constant pressure funnel and a condenser 3 I and THF, add CH dropwise at room temperature 3 SCH 3 , And fully stirred for 8h; then add sodium methoxide and stir for deprotonation reaction for 0.2h, ...
Embodiment 2
[0044] The preparation of the triazole modified Mannich base compound includes the following steps:
[0045] (1) Mix the 30% formaldehyde solution with diethanolamine and add it to the solvent ethanol, then adjust the pH to 5 with 25% hydrochloric acid, stir and raise the temperature to 40°C, react for 0.6h, continue to heat up At 70℃, add the acid component acetone dropwise, keep the temperature for 5.5h; distill under reduced pressure to remove the solvent, then wash with ether for 2 to 3 times, and dry to obtain Mannich base; among them, formaldehyde, diethanolamine and acetone in the formaldehyde solution The ratio of the amount of substances is 3:1.5:1, and the volume ratio of ethanol to formaldehyde solution is 30:1.
[0046] (2) Add CH to a 500mL three-necked flask equipped with an electric stirrer, a constant pressure funnel and a condenser 3 I and solvent THF, add CH dropwise at room temperature 3 SCH 3 , And fully stirred for 12h; then add sodium ethoxide and stir for dep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com