Citicoline and synthetic method thereof
A technology of citicoline and its synthesis method, which is applied in the new field of synthesis of pharmaceutical molecules, and can solve problems such as affecting product quality, difficult separation, and difficult removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
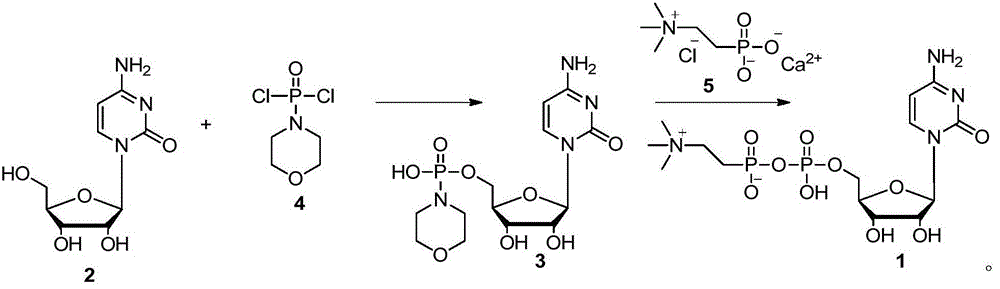
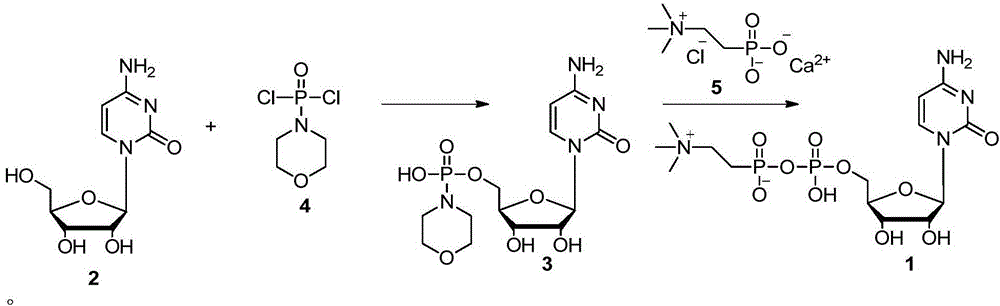
[0017] Add dichloromethane (50mL) into the three-necked flask, add phosphorus oxychloride (1.8mL, 20mmol), cool down to 0-5°C, add dropwise morpholine (2.6mL, 30mmol), and control the dropping time at 30min Within a period of time, the dropwise addition was completed, the temperature was raised, and the temperature was kept at room temperature (25-30° C.), and the stirring reaction was continued for 2 h. Atmospheric pressure distills out dichloromethane, and then fractionates under reduced pressure to collect distillates with a boiling range of 124-126°C (1.33KPa) to obtain phosphoromorpholine dichloride.
[0018] Add cytidine (10g, 41.1mmol) into a three-necked flask, add solvent (50mL), stir, cool down to 0-5°C, add dichlorophosphorylmorpholine (10.0g, 49.3mmol) dropwise, and dropwise The time is within 30 minutes, the dropwise addition is completed, the temperature is raised, and the temperature is kept at room temperature (25-30° C.), and the reaction is continued to stir ...
Embodiment 2
[0021] Add 5'-phosphorylmorpholinocytidine (500 g, 1.3 mol) to methanol (2 L), add calcium phosphorylcholine chloride (370 g, 1.5 mol), add 0.13 mol of sulfuric acid, and keep the temperature at room temperature ~ 60 Any temperature between ℃, stirred and reacted for 15h, distilled off the solvent under reduced pressure, and the condensed product of 5'-phosphorylmorpholinocytidine and calcium phosphorylcholine chloride was dissolved in 1L of absolute ethanol under reflux, Cool down naturally, gradually add 200mL of water dropwise to the solution, citicoline can be precipitated. The preparation of the 5'-phosphorylmorpholinocytidine is the same as in Example 1.
Embodiment 3
[0023] Add cytidine (100g, 0.4mol) into a three-necked flask, add pyridine (500mL), stir, cool down to 0-5°C, add dichlorophosphorylmorpholine (100g, 0.5mol) dropwise, and control the dropping time The dropwise addition was completed within 30 minutes, the temperature was raised, and the temperature was kept at room temperature (25-30° C.), and the stirring reaction was continued for 2 hours. After the reaction was completed, 500 mL of saturated aqueous sodium bicarbonate solution was added and stirred for 20 minutes to consume excess dichlorophosphorylmorpholine. Suction filtration, the filtrate was collected, and the solvent was distilled off under reduced pressure to obtain a light yellow oil, which was recrystallized from absolute ethanol to obtain 5'-phosphorylmorpholinocytidine as a white solid.
[0024]Add 5'-phosphorylmorpholinocytidine (500 g, 1.3 mol) to methanol (2 L), add calcium phosphorylcholine chloride (370 g, 1.5 mol), add 0.13 mol of sulfuric acid, and keep t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com