A-A type conjugated polymer based on isoindigo-blue and preparation method and application thereof
A conjugated polymer, A-A technology, applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve problems such as high cost, difficult preparation and purification, and low light absorption coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This embodiment provides an A-A type conjugated polymer based on isoindigo, and the general structural formula is shown in formula (I);
[0046] Formula (I)
[0047] Wherein, R is a C1-C32 alkane group, a C2-C32 alkene group, a C2-C32 alkynyl group, an etheroxy group, an alkyl chain amine or a mercapto group; n is an integer greater than or equal to 4 and less than or equal to 100;
[0048] Ar is an aromatic compound fragment, specifically:
[0049]
[0050] Therefore, the chemical formula of the A-A type conjugated polymer of this embodiment is specifically shown in formula (II), formula (III) or formula (IV);
[0051] formula (II);
[0052] formula (III);
[0053] Formula (IV).
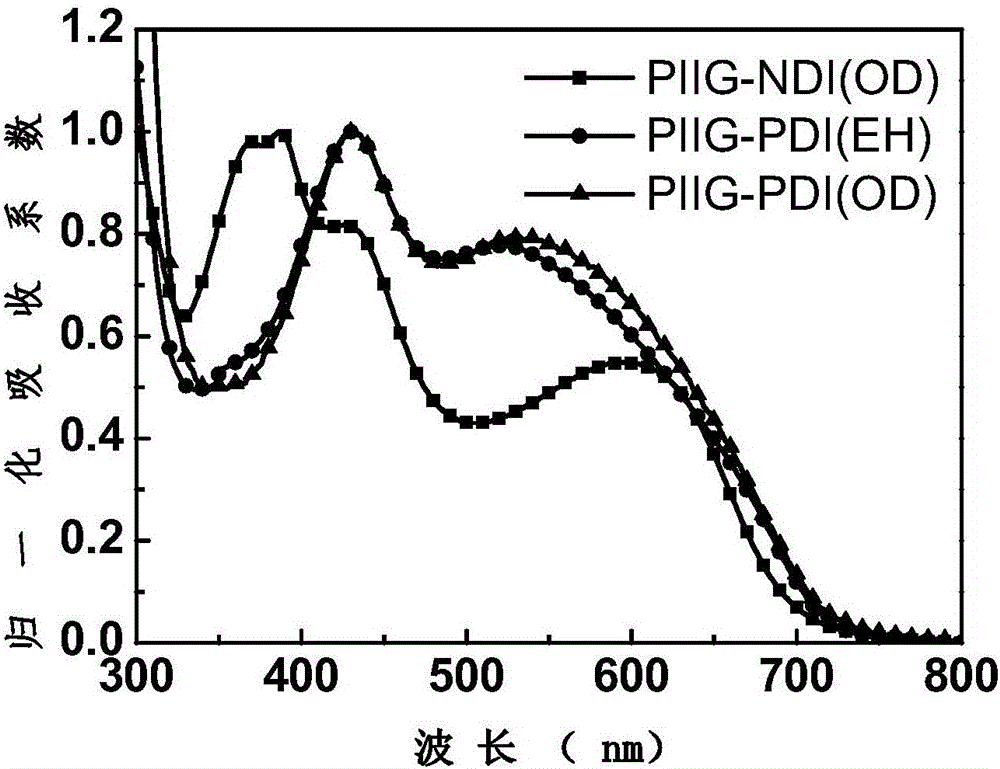
[0054] The conjugated polymer of formula (II) is designated as PIIG-NDI(OD), the conjugated polymer of formula (III) is designated as PIIG-PDI(OD), and the conjugated polymer of formula (IV) is designated as PIIG-PDI (EH). exist figure 1 The UV-Vis absorption spectra of PIIG...
Embodiment 2
[0056] This example provides a method for preparing the A-A type conjugated polymer PIIG-NDI(OD) represented by the above formula (II).
[0057] 6,6'-(N,N'-2-octyldodecyl)-Pinacoldiboronisoindigo and N,N'-bis were catalyzed by tris(dibenzylideneacetone)dipalladium and tris(o-methylphenyl)phosphorus (2-octyldodecyl)-2,6-dibromonaphthalene-1,4,5,8-bis(dicarboximide) was reacted in an organic solvent at 90°C-100°C for 70 hours-72 hours to obtain the conjugate of the above formula (II) polymer.
[0058] In this method, 6,6'-(N,N'-2-octyldodecyl)-Pinacoldiboronisoindigo and N,N'-bis(2-octyldodecyl)-2,6-dibromonaphthalene-1,4,5,8-bis( dicarboximide) in a molar ratio of 1:0.99-1.05, preferably 1:1. The dosage of tris(dibenzylideneacetone)dipalladium is 1%-2% of the molar dosage of 6,6'-(N,N'-2-octyldodecyl)-Pinacoldiboronisoindigo, and the dosage of tris(o-methylphenyl)phosphorus It is 5%-6% of the molar amount of 6,6'-(N,N'-2-octyldodecyl)-Pinacoldiboronisoindigo. The organic so...
Embodiment 3
[0062] The third embodiment provides a specific method for the A-A type conjugated polymer PIIG-NDI(OD) represented by the above formula (II).
[0063]
[0064] First of all, in the above formula, the place marked 1 is compound 1, and the place marked 2 is compound 2.
[0065] Under nitrogen protection, into a 25ml Schlenk tube, add 109.7mg (0.102mmol) of compound 1, 100.5mg (0.102mmol) of compound 2, 108.5mg (0.5mmol) of potassium phosphate, 50mg of tetrabutylammonium chloride , 1.4 mg (1.5 mol%) of Pd 2 (dba) 3 , 1.9 mg (6 mol%) of P-(o-Tol) 3 , 7ml of toluene, 1.5ml of water. The reaction was stirred at 90°C for 3 days.
[0066] It was then capped with pinacol phenylboronic acid and bromobenzene.
[0067] Then the product PIIG-NDI (OD) 116.3 mg was obtained from chloroform by Soxhlet extraction with a yield of 69%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com