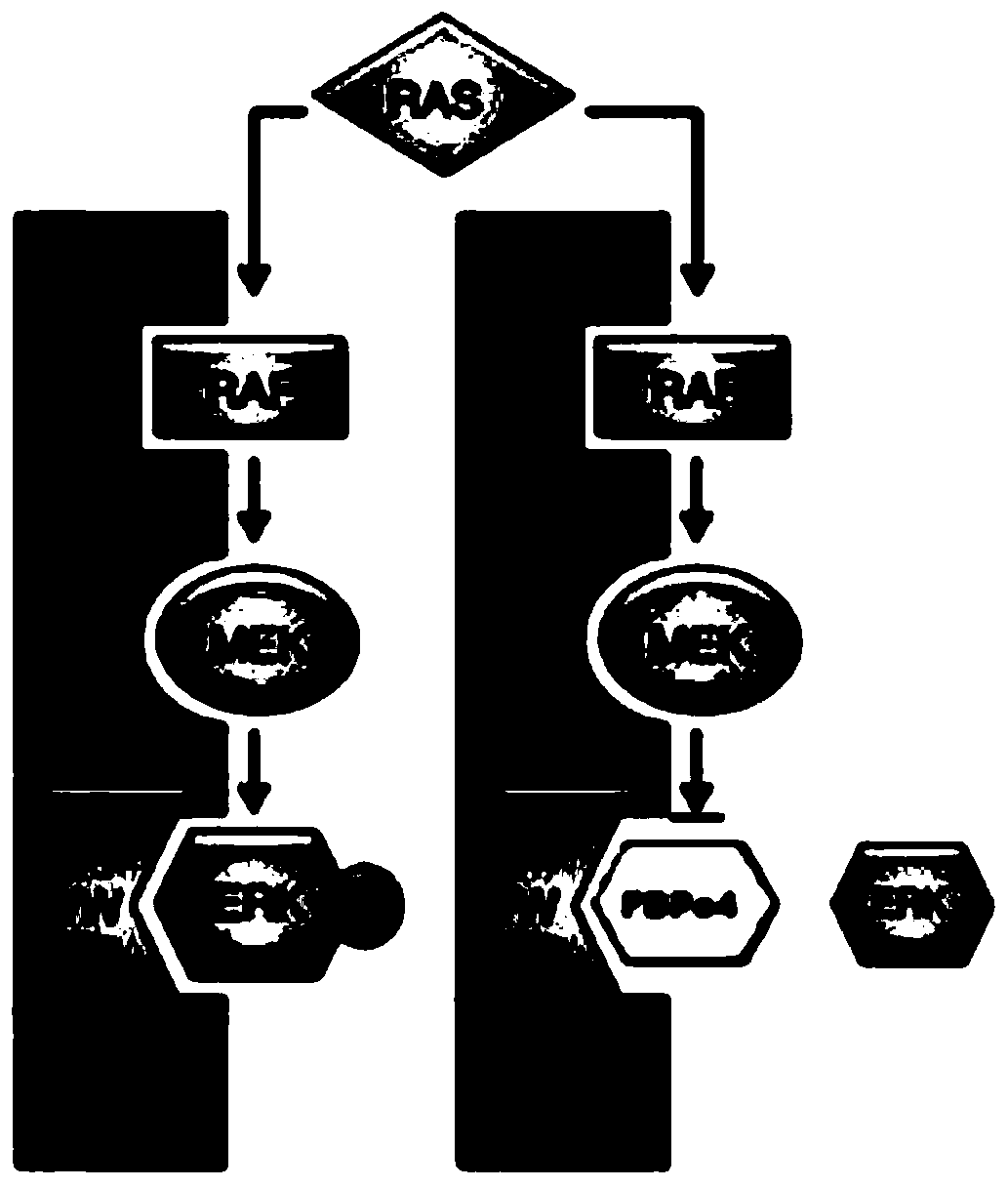
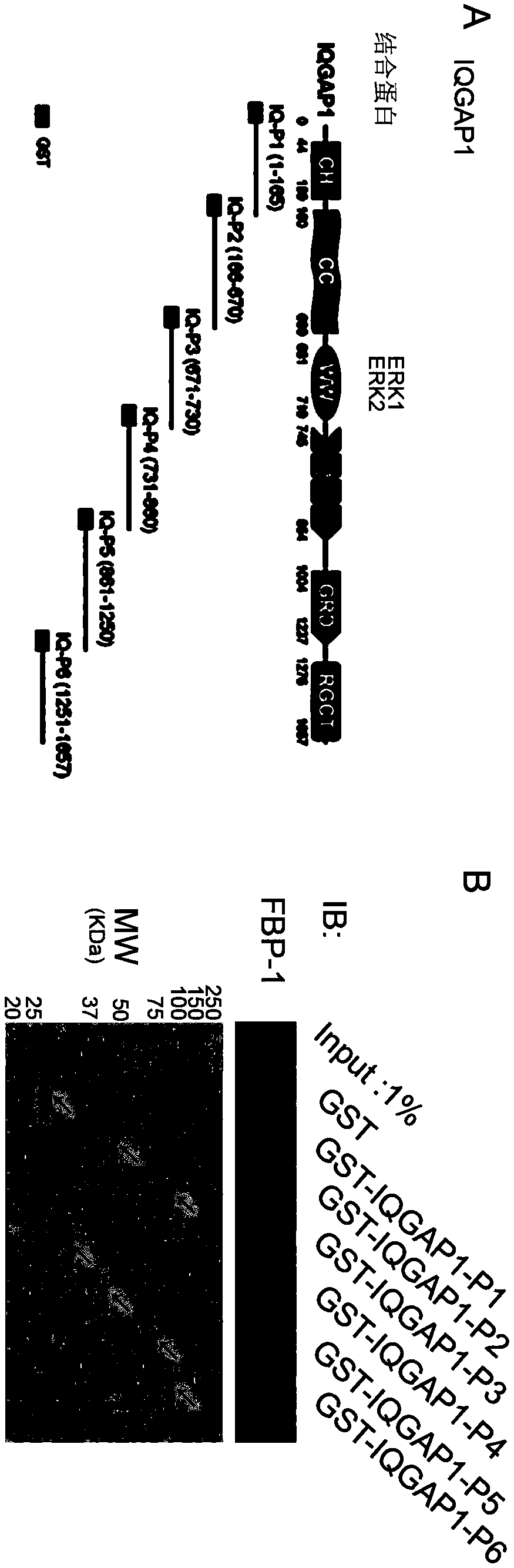
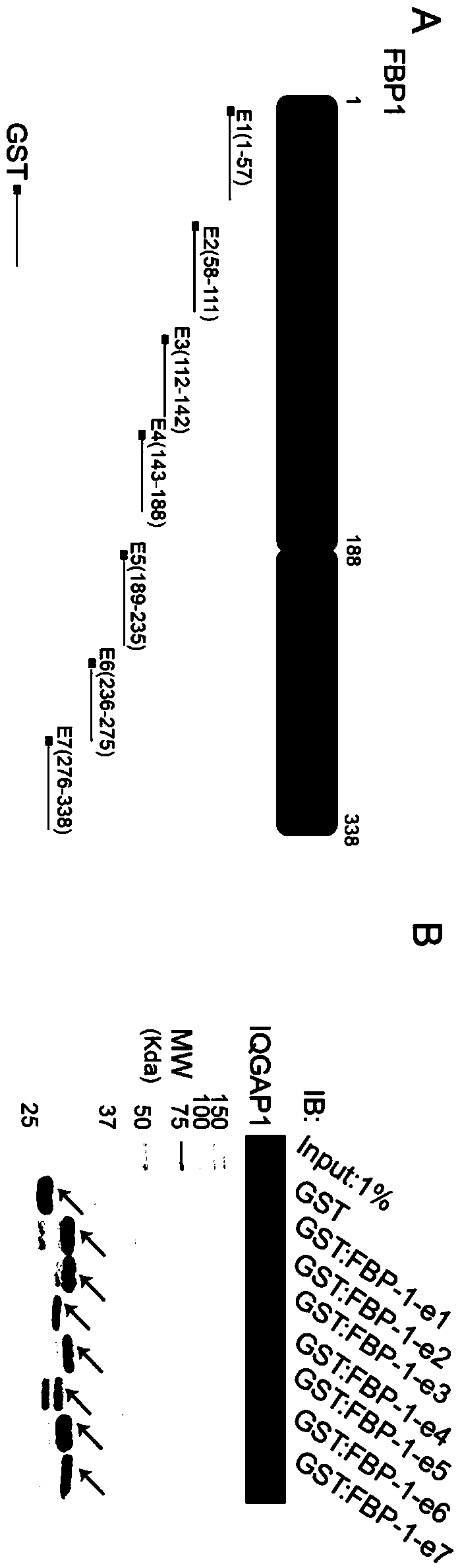
A kind of erk1/2 protein inhibitory polypeptide, its preparation method and application
A protein inhibition, recombinant plasmid technology, applied in the field of bioengineering, can solve the problems of restricting ERK1/2 protein research, influence, and no specific ERK1/2 inhibitor, etc., to achieve in vivo research, broad application prospects, cell less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 contains the interaction verification and preliminary functional verification of FBPe4 and IQGAP1
[0042] S11. Main experimental materials:
[0043] 1), cells
[0044] In this experiment, the pancreatic cancer cell line PANC-1 was selected. The pancreatic cancer cell line is a K-ras mutant cell line, and pERK1 / 2 can be detected under normal conditions, so the changes of pERK1 / 2 can be observed after FBPe4 treatment, which is convenient for observing the experimental effect.
[0045] PANC-1 was purchased from ATCC (American Type Culture Collection, Manassay, VA, USA). PANC-1 cells used DMEM medium (CORNING, Manassa, VA, USA) containing 10% fetal bovine serum (Life Technologies), 100 units / ml penicillin and 100 μg / ml streptomycin (Life Technologies). Cultured in a cell incubator with a culture condition of 5% CO 2 , 37°C, 95% humidity.
[0046] 2), plasmid
[0047] Flag-FBP1, FBPe4 and FBPΔE4 were loaded into pcDNA3.1 / V5-His-TOPO (Invitrogen, 460083) vect...
Embodiment 2
[0070] Embodiment 2 Containing the construction of the recombinant plasmid of FBPe4 and the expression of FBPe4
[0071] According to the base sequence shown in SEQ ID No: 2, this sequence was cloned into the expression vector pcDNA3.1 / V5-His-TOPO (containing Fag tag, which can be detected by Flag antibody).
[0072] S21. Amplify the target DNA by PCR, and recover the target DNA by electrophoresis
[0073] Utilize the FBP1 plasmid (sequence shown in SEQ ID No:3) as template amplification to obtain the gene sequence of FBPe4 (i.e. SEQ ID No:2), the PCR amplification reaction system is:
[0074]
[0075] Among them, the FBPe4 upstream primer is:
[0076] 5-CGGATATCAAGGATGCTCTGCAACCAGGCCGG-3';
[0077] FBPe4 downstream primers:
[0078] 5'-CGCTCGAGGGCAAGGACCAGCATGGTGGCACT-3'.
[0079] The PCR reaction procedure is:
[0080]
[0081] After the completion, the amplified product was subjected to agarose electrophoresis, and the target band of about 100 bp was cut out, and t...
Embodiment 3
[0101] Example 3 FBPe4 inhibits the phosphorylation of ERK1 / 2
[0102] S31. Using the pcDNA3.1 / V5-His-TOPO-FBPe4 recombinant plasmid to construct the FBP1G260R plasmid.
[0103] Primers constructed by FBP1G260R (glycine at position 260 replaced with arginine):
[0104] Upstream primers:
[0105] 5'-CATCGCACTCTGGTCTACAGAGGGATATTTCTGTAC-3'
[0106] Downstream primers:
[0107] 5'-AACATCAGCCACCATGGAGCCCACATACCGGGCCC-3'
[0108] FBP1G260R lost the enzymatic activity of synthesizing glycogen.
[0109] The structures of FBP1G260R and FBP1ΔFBPe4 mutant proteins are as follows: image 3 As shown in A.
[0110] S32. The FBP1G260R and FBP1ΔFBPe4 plasmids were transformed into PANC-1 cells and expressed, and the cell lysate was collected and used for co-immunoprecipitation experiments with Flag antibody (the recombinant proteins expressed by FBP1G260R and FBP1ΔFBPe4 plasmids all had Flag tags).
[0111] The experimental results show that FBP1, FBP1G260R, and FBPe4 can ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com