Method for recovering copper from copper dust and immobilizing arsenic from copper dust into scorodite
A technology for recovering copper and scorodite, applied in chemical instruments and methods, inorganic chemistry, iron compounds, etc., can solve the problems of low process recovery rate, high recovery cost, and potential environmental protection hazards, and achieve low arsenic content, high recovery Low cost and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
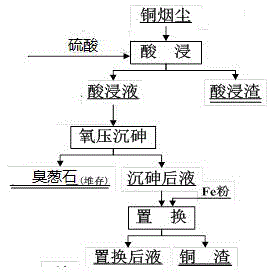
[0053] 1). Acid leaching the copper fumes to obtain an acid leaching solution. Take 700g of copper fume (Fe is 2~12%, As is 4~12%, Cu is 3~6%, Zn is 7~10%, Pb is 15~26.49%), according to the liquid-solid ratio (mL:g) It is 5.7:1, and the acidity of sulfuric acid is 30g / L, at room temperature (18~22°C), and the reaction time is 2h. The leaching indicators are: the leaching rate of zinc from slag is 97.54%, the leaching rate of copper is 82.83%, and the leaching rate of arsenic is 73.05%.
[0054] 2). At 140°C, the mass ratio of iron to arsenic is 1.5:1, the arsenic concentration is 18g / l, the pH is 4.5, the copper ion concentration is 0.1g / L, the zinc concentration is 75g / L, and the oxygen partial pressure is 1.3MPa , under the condition that the reaction time is 2h, oxygen pressure precipitation of arsenic is carried out on the pickling solution to obtain scorodite. The index of oxygen pressure precipitation arsenic: the precipitation rate of copper is 0.6%, the precipitatio...
Embodiment 2
[0057] 1). Acid leaching the copper fumes to obtain an acid leaching solution. Take 700g of copper fume (Fe is 2~12%, As is 4~12%, Cu is 3~6%, Zn is 7~10%, Pb is 15~26.49%), according to the liquid-solid ratio (mL:g) It is 5.7:1, and the acidity of sulfuric acid is 22g / L, at room temperature (18~22°C), and the reaction time is 1.5h. The leaching indicators are: the leaching rate of zinc from slag is 91%, the leaching rate of copper is 78.21%, and the leaching rate of arsenic is 65.29%.
[0058] 2). At 150°C, the mass ratio of iron to arsenic is 1, the concentration of arsenic is 5g / l, the acidity of sulfuric acid is 10g / L, the concentration of copper ions is 2g / L, the concentration of zinc is 30g / L and the partial pressure of oxygen is 0.7MPa. Under the condition that the time is 2.5 hours, oxygen pressure precipitation of arsenic is carried out on the pickling solution to obtain scorodite. Indicators of oxygen pressure precipitation arsenic: copper precipitation rate 0.8%, ...
Embodiment 3
[0061] 1). Acid leaching the copper fumes to obtain an acid leaching solution. Take 700g of copper fume (Fe is 2~12%, As is 4~12%, Cu is 3~6%, Zn is 7~10%, Pb is 15~26.49%), according to the liquid-solid ratio (mL:g) It is 5.7:1, and the acidity of sulfuric acid is 14g / L, at room temperature (18~22°C), and the reaction time is 2h. The leaching indicators are: the leaching rate of zinc from slag is 86%, the leaching rate of copper is 76%, and the leaching rate of arsenic is 58.23%.
[0062] 2). At 160°C, the mass ratio of iron to arsenic is 2, the concentration of arsenic is 30g / l, the acidity of sulfuric acid is 20g / L, the concentration of copper ions is 8g / L, the concentration of zinc is 75g / L, and the partial pressure of oxygen is 0.1MPa , under the condition that the reaction time is 3h, oxygen pressure precipitation of arsenic is carried out on the pickling solution to obtain scorodite. Indicators of oxygen pressure precipitation arsenic: copper precipitation rate 1.0%, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com