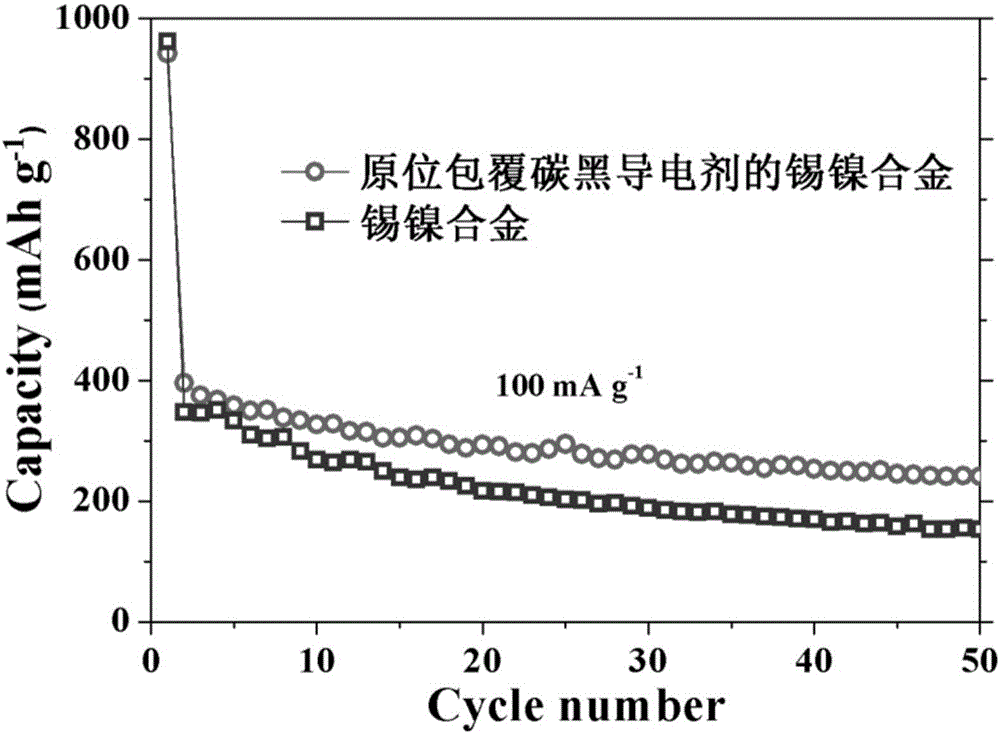
Preparation method of in-situ carbon conductive agent-coated tin-nickel alloy and application of in-situ carbon conductive agent-coated tin-nickel alloy as cathode material for sodium-ion battery
A tin-nickel alloy, in-situ coating technology, applied in the direction of battery electrodes, secondary batteries, circuits, etc., can solve the problem of failure to fully utilize the buffering and conductive effects of carbon conductive agents, and the difficulty in achieving uniform distribution of active materials and carbon conductive agents and other issues to achieve the effect of improving structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) tin tetrachloride and potassium nickel cyanide are dissolved in the aqueous solution of the carbon black conductive agent that is dispersed mass concentration respectively at 10 mg / ml, and form concentration is 0.2 mol / liter of tin tetrachloride and 0.2 mol / ml The aqueous solution of potassium nickel cyanide of 1 liter; then the aqueous solution of tin tetrachloride and potassium nickel cyanide is mixed to form the Sn(IV)–Ni(II) cyano coordination polymer hydraulic coagulation of in-situ fixed carbon black conductive agent Glue; Wherein, the molar ratio of tin tetrachloride and potassium nickel cyanide is 1:1.
[0027] (2) The Sn(IV)-Ni(II) cyano coordination polymer hydrogel of the in-situ fixed carbon black conductive agent obtained in step (1) is used as a precursor, and sodium borohydride is added therein as a reducing agent, The molar ratio of sodium borohydride to tin tetrachloride is 20:1, react for 1 hour, wash and dry the product, and obtain a tin-nickel al...
Embodiment 2
[0030] (1) tin tetrachloride and potassium nickel cyanide are dissolved in the aqueous solution of the carbon black conductive agent that disperses mass concentration respectively in 1 mg / ml, form the tin tetrachloride that concentration is 0.1 mol / liter and 1 mol / ml The aqueous solution of potassium nickel cyanide of 1 liter; then the aqueous solution of tin tetrachloride and potassium nickel cyanide is mixed to form the Sn(IV)–Ni(II) cyano coordination polymer hydraulic coagulation of in-situ fixed carbon black conductive agent Glue; Wherein, the molar ratio of tin tetrachloride and potassium nickel cyanide is 0.1:1.
[0031] (2) The Sn(IV)-Ni(II) cyano coordination polymer hydrogel of the in-situ fixed carbon black conductive agent obtained in step (1) is used as a precursor, and sodium borohydride is added therein as a reducing agent, The molar ratio of sodium borohydride to tin tetrachloride is 100:1, react for 24 hours, wash and dry the product, and obtain a tin-nickel a...
Embodiment 3
[0034] (1) dissolving tin tetrachloride and potassium nickel cyanide in the aqueous solution of the carbon black conductive agent that dispersed mass concentration is 100 mg / ml respectively, forming concentration is 5 mol / liter of tin tetrachloride and 0.5 mol / ml The aqueous solution of potassium nickel cyanide of 1 liter; then the aqueous solution of tin tetrachloride and potassium nickel cyanide is mixed to form the Sn(IV)–Ni(II) cyano coordination polymer hydraulic coagulation of in-situ fixed carbon black conductive agent Glue; Wherein, the molar ratio of tin tetrachloride and potassium nickel cyanide is 10:1.
[0035] (2) The Sn(IV)-Ni(II) cyano coordination polymer hydrogel of the in-situ fixed carbon black conductive agent obtained in step (1) is used as a precursor, and sodium borohydride is added therein as a reducing agent, The molar ratio of sodium borohydride to tin tetrachloride is 1:1, react for 0.1 hour, wash and dry the product, and obtain a tin-nickel alloy co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com