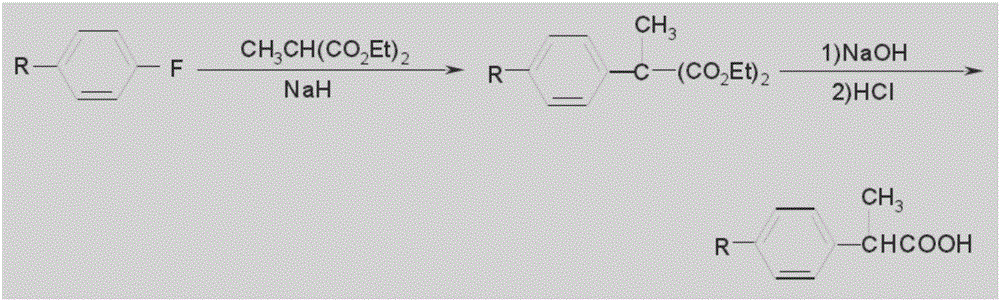
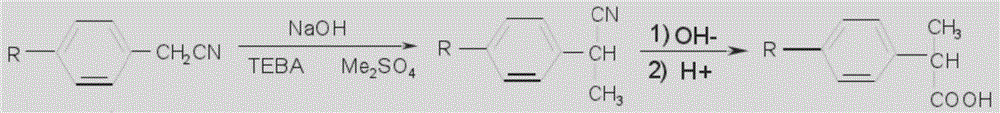
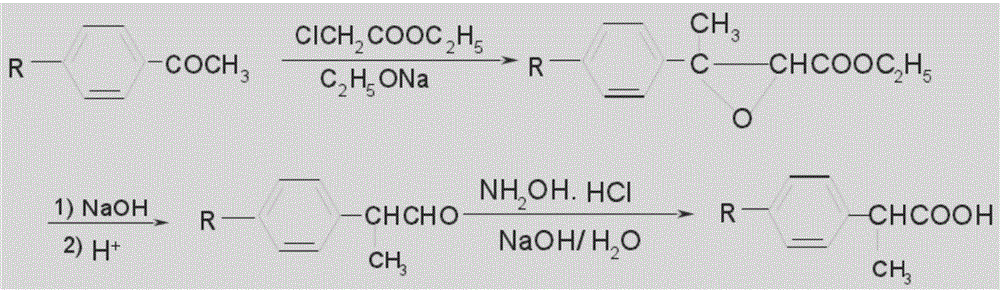
Method for preparing loxoprofen intermediate
An intermediate and system technology, applied in the field of preparation of loxoprofen intermediates, can solve the problems of poor product selectivity, low conversion rate, many by-products, etc., and achieve low production cost, high conversion rate, and few by-products. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The present embodiment loxoprofen intermediate preparation method, concrete steps are as follows:
[0071] 1) Preparation stage of 2-(phenylcyano)sodium propionate
[0072] Add 10g, 10g, 5.0g, and 45g of phenylacetonitrile, DMC, sodium methoxide, and toluene into the drying reactor, stir and heat up to 60°C, and keep the temperature for 1 hour; The product methanol was distilled off until no methanol was left, and the reaction solution was sampled and tested for later use.
[0073] 2) Preparation stage of methyl 2-(phenylcyano)propionate
[0074] Lower the temperature of the upper reaction solution to 35°C, control the reaction temperature at 35°C and add 14g of DMS dropwise at a constant speed. water, stirred, dissolved and separated into layers, and the toluene was recovered by distillation under reduced pressure until no toluene was evaporated to obtain methyl 2-(phenylcyano)propionate;
[0075] 3) 2-phenylpropionitrile preparation stage
[0076] 12g of 30% liqui...
Embodiment 2
[0082] The present embodiment loxoprofen intermediate preparation method, concrete steps are as follows:
[0083] 1) Preparation stage of 2-(phenylcyano)sodium propionate
[0084] Add 10g, 15g, 6.0g, and 50g of phenylacetonitrile, DMC, sodium methoxide, and toluene into the drying reactor, stir and heat up to 100°C, and keep warm for 5.5 hours; The product methanol was distilled off until no methanol was left, and the reaction solution was sampled and tested for later use.
[0085] 2) Preparation stage of methyl 2-(phenylcyano)propionate
[0086] Lower the temperature of the upper reaction solution to 35°C, control the reaction temperature at 85°C and add 10g of DMS dropwise at a constant speed. water, stirred, dissolved and separated into layers, and the toluene was recovered by distillation under reduced pressure until no toluene was evaporated to obtain methyl 2-(phenylcyano)propionate;
[0087] 3) 2-phenylpropionitrile preparation stage
[0088] Mix 11g of 30% liquid c...
Embodiment 3
[0094] The present embodiment loxoprofen intermediate preparation method, concrete steps are as follows:
[0095] 1) Preparation stage of 2-(phenylcyano)sodium propionate
[0096] Add phenylacetonitrile, DMC, a mixture of sodium methoxide and methanol (the content of sodium methoxide is 30%), and toluene respectively 10g, 12.5g, 18.33g, and 47.5g into the drying reactor, stir and heat up to 20°C, and keep warm for 10 Hours; after the reaction, the by-product methanol was distilled at 80°C under normal pressure until no methanol was distilled out, and the reaction solution was sampled and tested for use.
[0097] 2) Preparation stage of methyl 2-(phenylcyano)propionate
[0098] Lower the temperature of the upper reaction solution to 35°C, control the reaction temperature at 60°C and add 10g of DMS dropwise at a constant speed. water, stirred, dissolved and separated into layers, and the toluene was recovered by distillation under reduced pressure until no toluene was evaporat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com