A kind of mbp fusion heparanase Ⅱ and its coding gene and preparation method
A technology of heparanase and coding sequence, which is applied in the field of MBP fusion heparanase II and its coding gene and preparation, can solve the obvious effect of catalytic performance, and the catalytic ability and thermal stability of heparanase II enzyme have not been significantly improved and other problems, to achieve the effect of improving catalytic activity and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
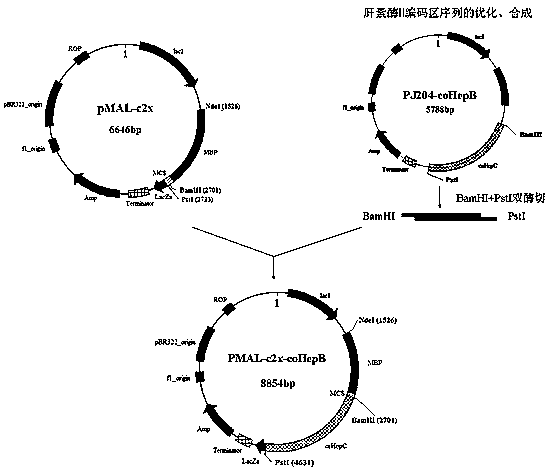
[0039]Example 1 Optimization of rare codons of heparinase II and construction and expression of MBP fusion heparinase II vector
[0040] 1. Heparinase II rare codon optimization
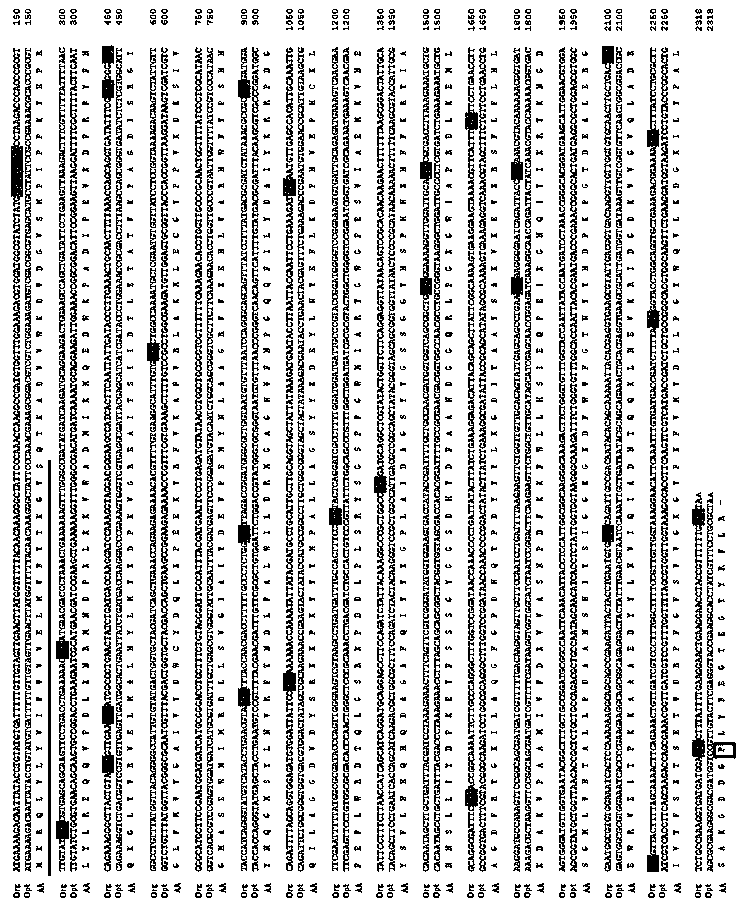
[0041] The coding region sequence of heparinase II from Flavobacterium heparin contains a large number of rare codons, which will seriously affect the protein translation efficiency when expressed in Escherichia coli. See figure 1 .
[0042] According to the codon usage preference of Escherichia coli, the coding region sequence of heparinase II was optimized with the current mainstream codon optimization software DNA2.0 (downloaded from https: / / www.dna20.com / official website). The protein sequence of heparinase II remains unchanged, and only the degeneracy of codons is used. Codons that are used less frequently in Escherichia coli and will affect the efficiency of ribosome passage during translation will be used with higher frequency. The codons were replaced to obtain the optimized heparinase II...
Embodiment 2
[0049] Example 2 Site-directed mutation and analysis of amino acid at 1126 site of MBP fusion heparinase II
[0050] 1. MBP fusion heparinase II 1126 site modification basis
[0051] The coding region sequences of heparinase II from two strains of Flavobacterium heparin were analyzed, and there was only one amino acid difference between the two protein amino acid sequences at position 758: P758A. According to the above rare codon optimization method and the construction method of MBP fusion heparinase II vector, the MBP fusion heparinase II expression vector with only one amino acid site difference (P1126A) was constructed. The above two vectors were transformed and expressed to obtain MBP-HepB fusion proteins HepB (1126P) and HepB (1126A).
[0052] Protein extraction and purification process: 100ml of the target Escherichia coli fermentation bacteria liquid was collected and resuspended with 20ml of protein extraction buffer, ultrasonically disrupted the bacteria, the crushe...
Embodiment 3
[0070]Example 3 MBP-fused heparinase II has a similar action site to the heparinase II 1126 site in the MBP-fused heparinase II vector in Example 2
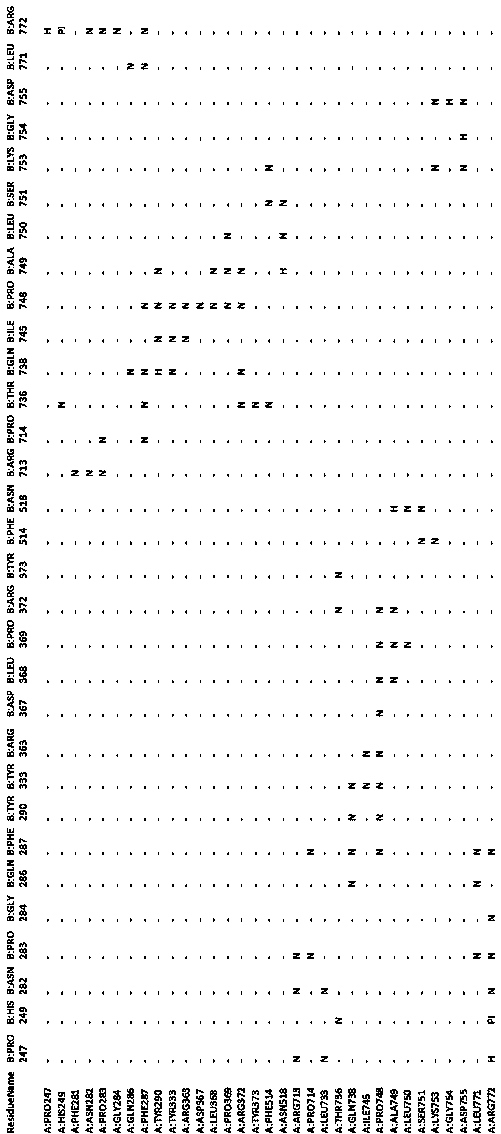
[0071] At present, there is no structural information of MBP-fused heparinase II in the PDB database, only the structural information of heparinase II. The 758 site of heparinase II was analyzed by Discover Studio 2.5, and this site is now on the binding surface of the heparinase II protein dimer (see Figure 7 ). Depend on Figure 7 and Figure 8 Comparative analysis showed that there was basically no difference in spatial conformation after amino acid substitution at position 758.
[0072] Using this method, the interaction force of the amino acids constituting the dimer binding surface was analyzed, and the results showed that among the 33 amino acid residues found to constitute the dimer binding surface, there were strong interactions between some amino acid residues. effect, some with slightly weaker interactions (see ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com