Mitochondria injection and application thereof
A technology of mitochondria and injection, applied in the field of biomedicine to achieve the effect of improving cell activity and increasing ATP production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. Preparation of mitochondrial intravenous injection for the treatment of drug-induced liver injury
[0029] Extraction of liver mitochondria: liver tissue was taken, and the animal was fasted for 12 hours before sampling, then quickly decapitated on ice, and 0.15 g of tissue was cut into 0.5 cm 2 Fragments, washed with washing solution (1mM Tris-HCl, pH7.0, 0.13MNaCl, 5mMKCl and 7.5mMMgCl 2 ) after washing, centrifuge at 800g for 10min; separate liquid (4mM Tris-HCl, pH7.4, 2.5mMNaCl and 0.5mMMgCl 2 ) resuspended, and allowed to stand for 1 to 2 minutes to collect the cells; crush the collected cells with a homogenizer to obtain a homogenate; transfer the homogenate to a centrifuge tube, and centrifuge at 4°C and 800×g for 5 minutes; collect Transfer the supernatant to a new centrifuge tube, centrifuge again at 4°C, 800×g for 5 minutes, and discard the precipitate; then transfer the supernatant to a new centrifuge tube, centrifuge at 4°C, 10,000×g for 10 minu...
Embodiment 2
[0036] Embodiment 2, preparation for treating Parkinson's disease mitochondrial injection
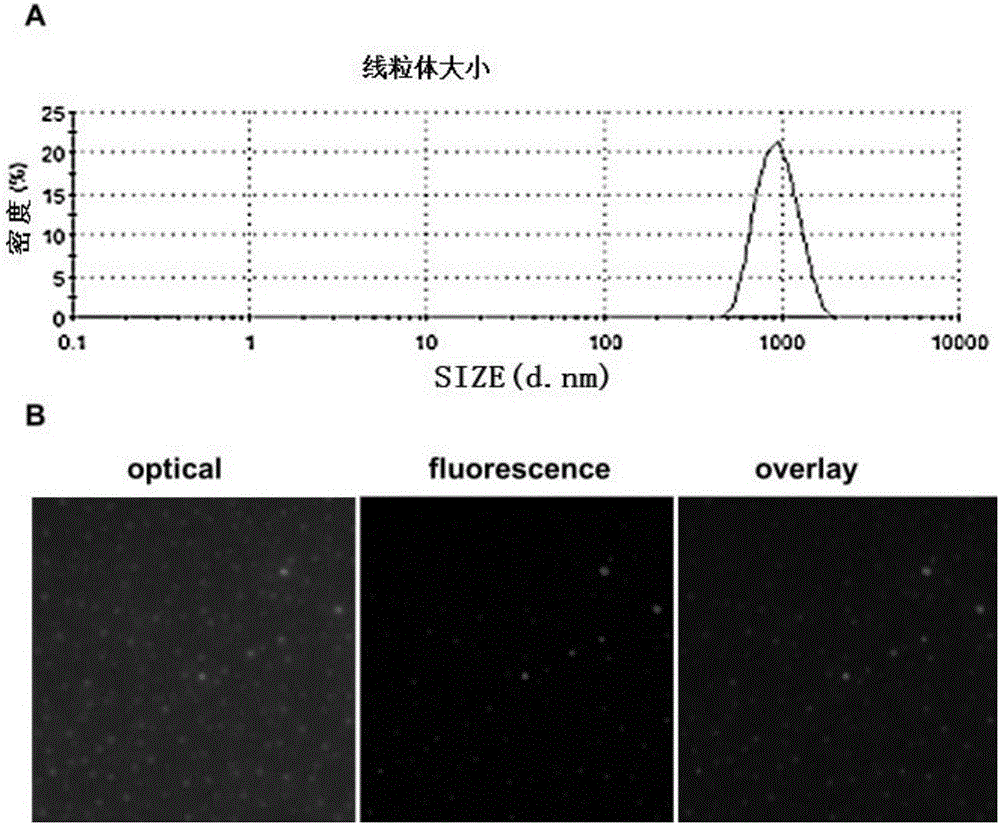
[0037] Extraction of brain mitochondria: take normal animal brain, homogenize with a glass homogenizer, transfer the homogenate to a centrifuge tube, centrifuge at 800×g for 5 min, collect the supernatant into a new centrifuge tube; transfer the supernatant to Centrifuge a new centrifuge tube at 10000×g for 10 min; then add 0.2 mL of mitochondrial extraction and purification solution (225 mM mannitol, 75 mM sucrose, 10 mM KCl, 10 mM Tris and pH7.0, 5 mM KH 2 PO 4 ) to resuspend the mitochondrial pellet, and centrifuge at 12000×g for 10 min; discard the supernatant, and the pellet is the mitochondria.
[0038] Mitochondria modification: disperse the extracted mitochondria with normal saline, slowly add the chitosan solution with a mass fraction of 2% to the mitochondrial solution at a mass ratio of 1:5, shake well, and store at room temperature (18-25°C) After standing for 5 minutes, a...
Embodiment 3
[0041] Embodiment 3, the application of mitochondrial injection
[0042] In order to verify that the mitochondrial injection prepared by the present invention can be used for intravenous injection, and can treat mitochondria-related diseases, the mitochondrial injection was subjected to the following experiments:
[0043] 1. The distribution of mitochondria in the body after intravenous injection
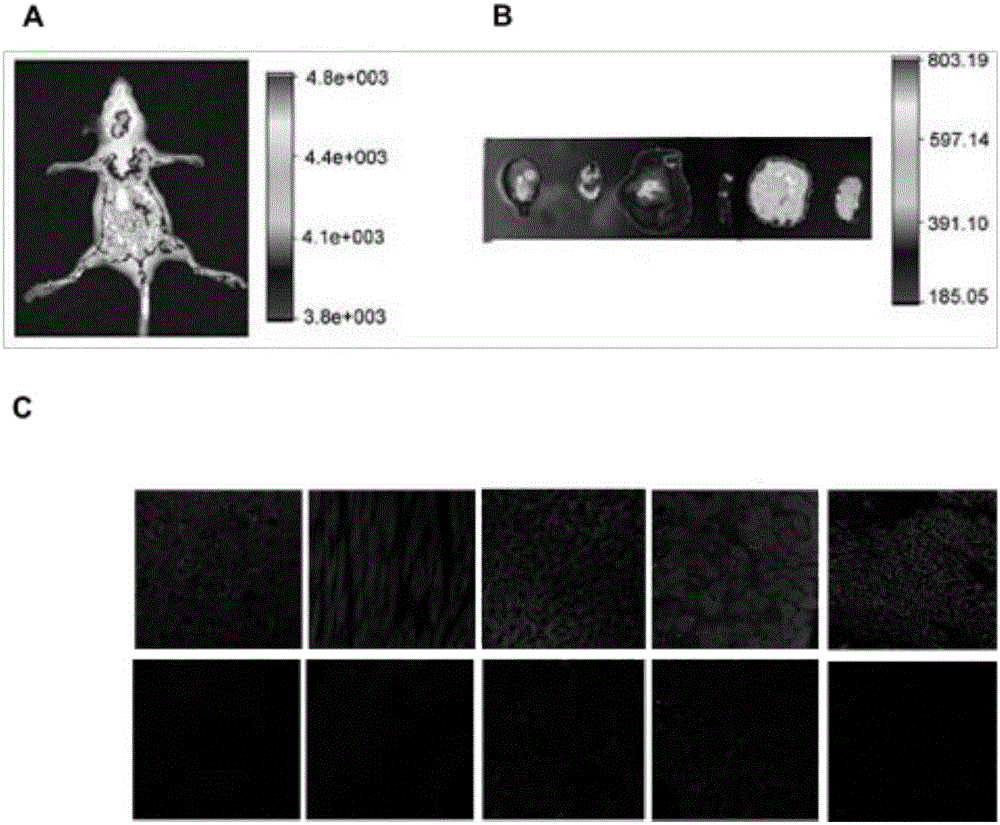
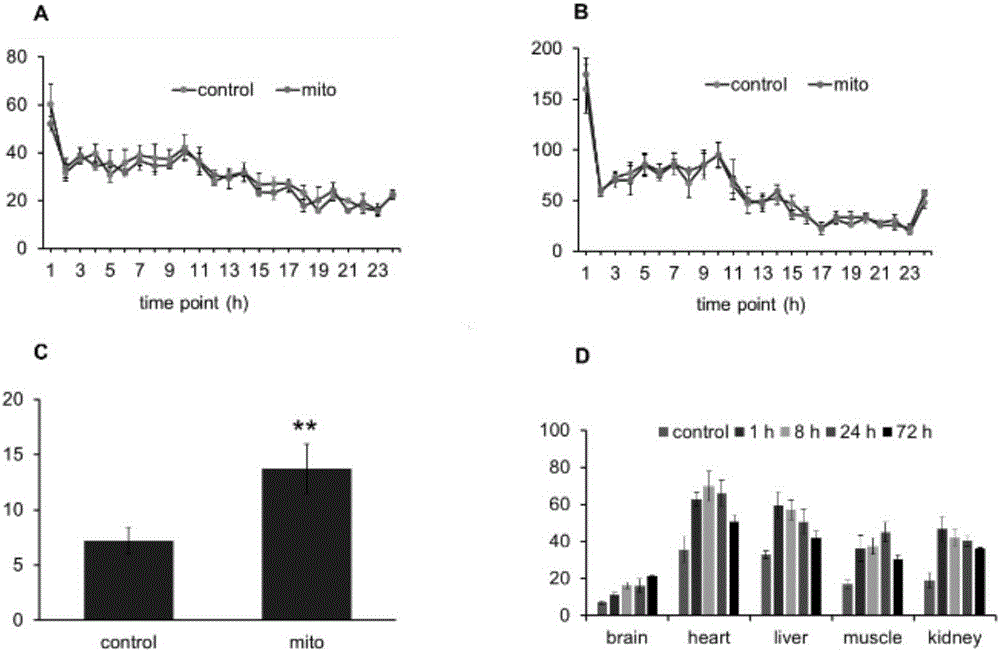
[0044] The mitochondrial injection prepared with normal saline (mitochondria were prepared for fluorescent staining) was injected into nude mice (0.5 mg / kg). After 2 hours of injection, the nude mice were anesthetized with 4% chloral hydrate by mass fraction and placed in Fluorescent imaging was performed in a small animal in vivo imager (Carestream Co.). Subsequently, the mice were perfused with ice-cold saline, and brain, liver, kidney, lung, skeletal muscle and other tissues were separated for tissue imaging. The results showed that there was a wide distribution of fluorescence ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com