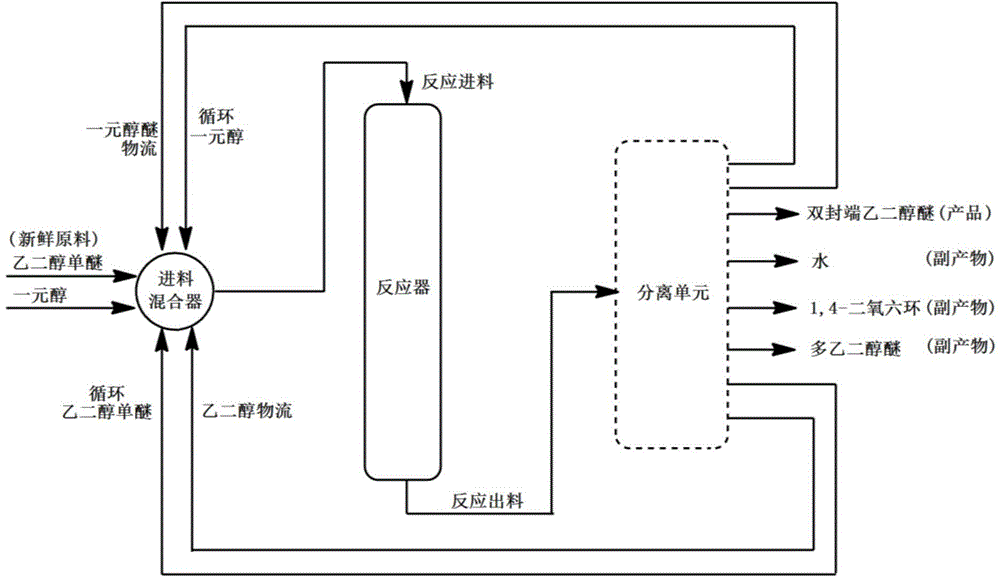
Preparation method for double-terminated glycol ether
A technology of glycol ether and ethylene glycol monoether, applied in the field of preparation of double-terminated glycol ether, can solve the problems of low catalyst yield, selectivity and life, difficult regeneration of resin catalyst, etc. The effect of raw material utilization rate, selectivity improvement, and low economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
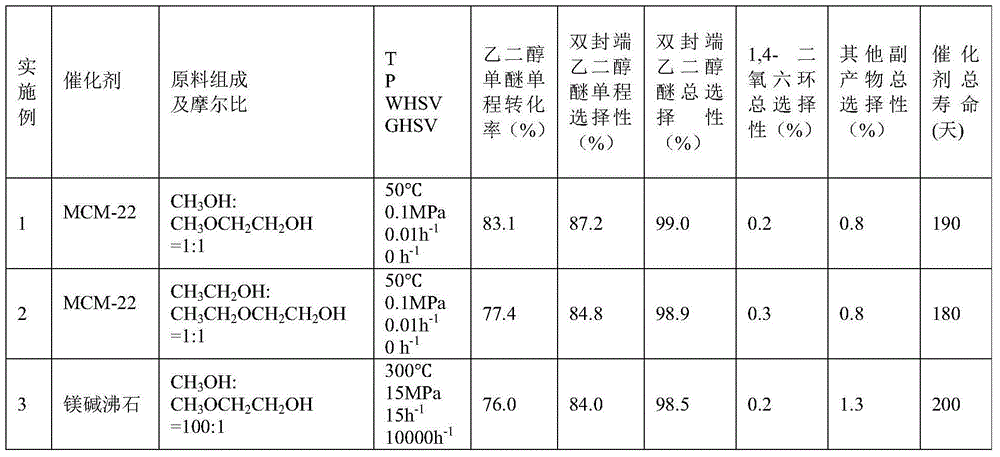
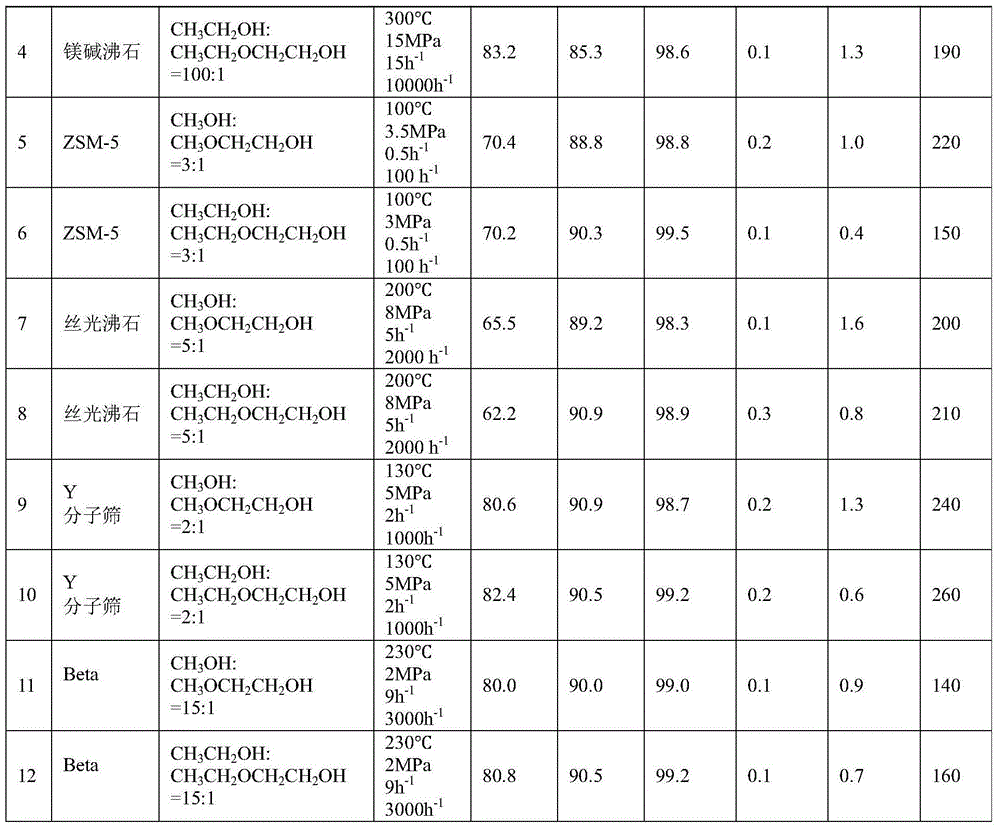
[0067] 2.0Kg of hydrogen-type MCM-22 molecular sieve catalyst with a silicon-aluminum ratio (Si:Al)=45:1 was extruded with alumina as a binder. ℃ for 5 hours to obtain a rod-shaped catalyst with a diameter of 3 mm, a length of 3 mm, and a mass content of alumina of 20%. Get 1.0Kg of this catalyst and put it into a stainless steel reaction tube with an internal diameter of 32mm, activate it with nitrogen at normal pressure and 550°C for 4 hours, then drop it to the reaction temperature (abbreviated as T)=50°C, the molar ratio of the fresh raw materials introduced for CH 3 OH:CH 3 OCH 2 CH 2 OH=1:1, reaction pressure (abbreviated as P)=0.1MPa, mass space velocity of ethylene glycol monoether in fresh raw materials (abbreviated as WHSV)=0.01h -1 , without carrier gas, after the reaction is stable, the product is analyzed by gas chromatography, and the conversion rate of ethylene glycol monoether and the single-pass selectivity of the product are calculated. Then, the monohyd...
Embodiment 2
[0069] Raw material and mol ratio among the embodiment 1 are changed into
[0070] CH 3 CH 2 OH:CH 3 CH 2 OCH 2 CH 2 OH=1:1, the rest of the experimental steps are consistent with Example 1. The reaction conditions and results are shown in Table 1.
Embodiment 3
[0072] The catalyst in Example 1 is replaced by a hydrogen-type ferrierite molecular sieve, Si:Al=15:1, T=300°C, P=15MPa, and the molar ratio of the feed material is CH 3 OH:CH 3 OCH 2 CH 2 OH=100:1, WHSV=15h -1 , the carrier gas is nitrogen, the carrier gas nitrogen volume space velocity (abbreviated as GHSV) = 10000h -1 , the rest of the experimental steps are consistent with Example 1. The reaction conditions and results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com