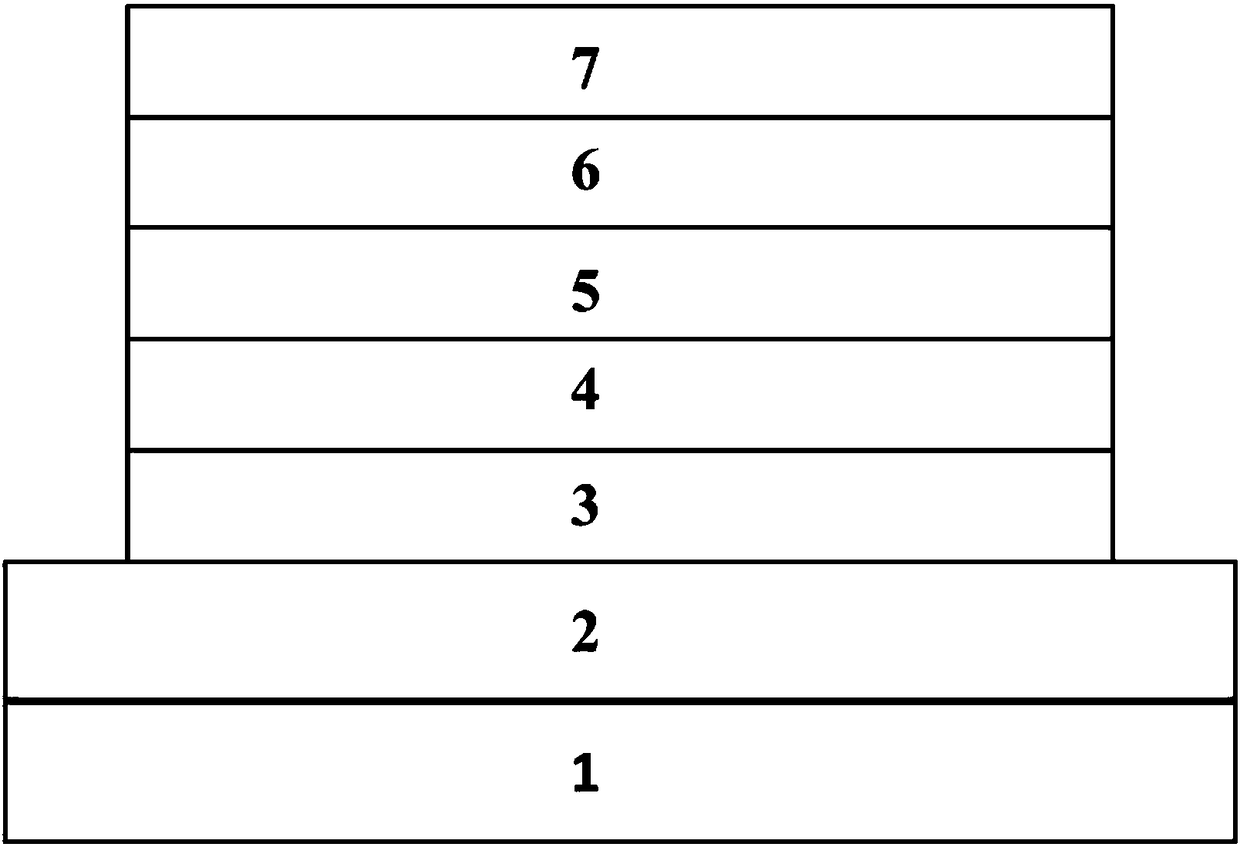
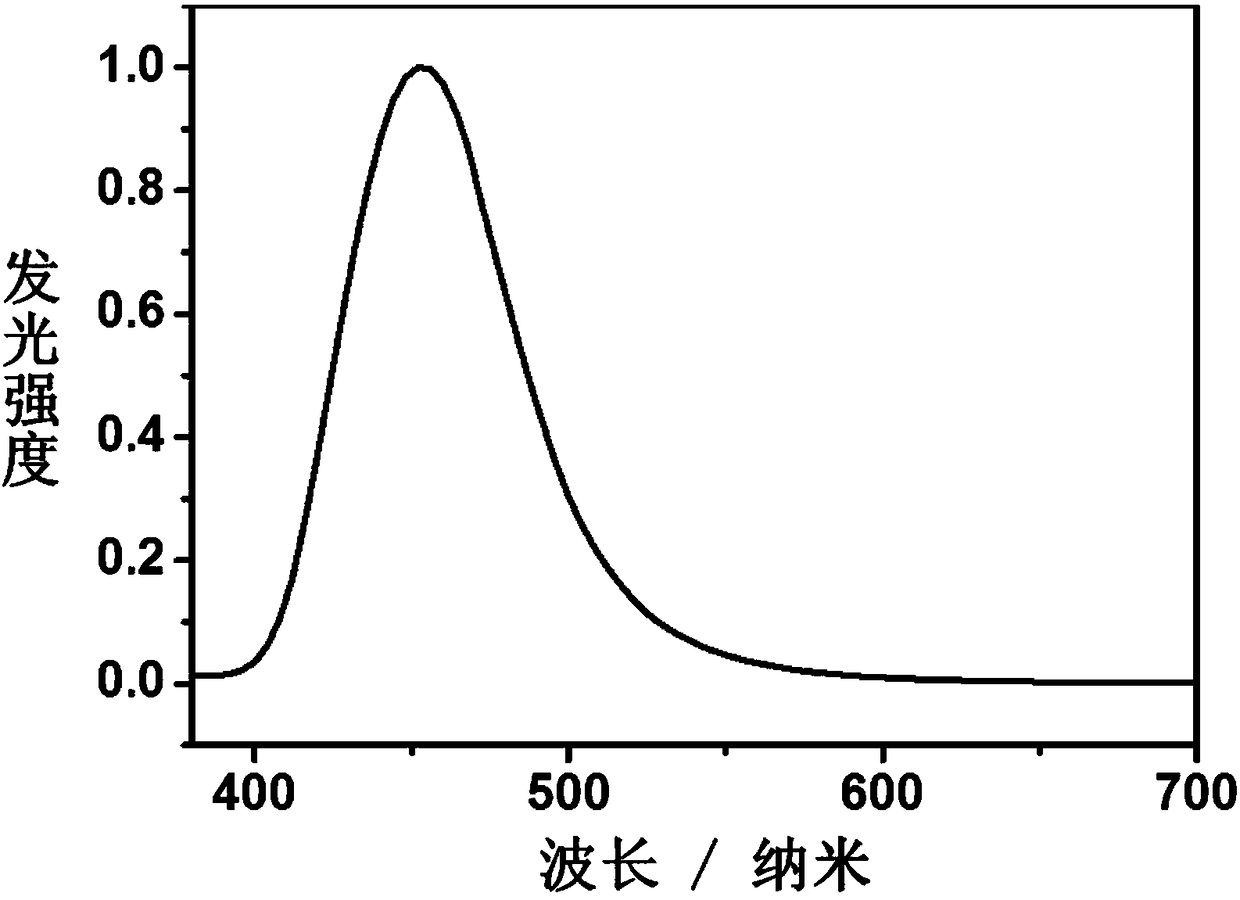
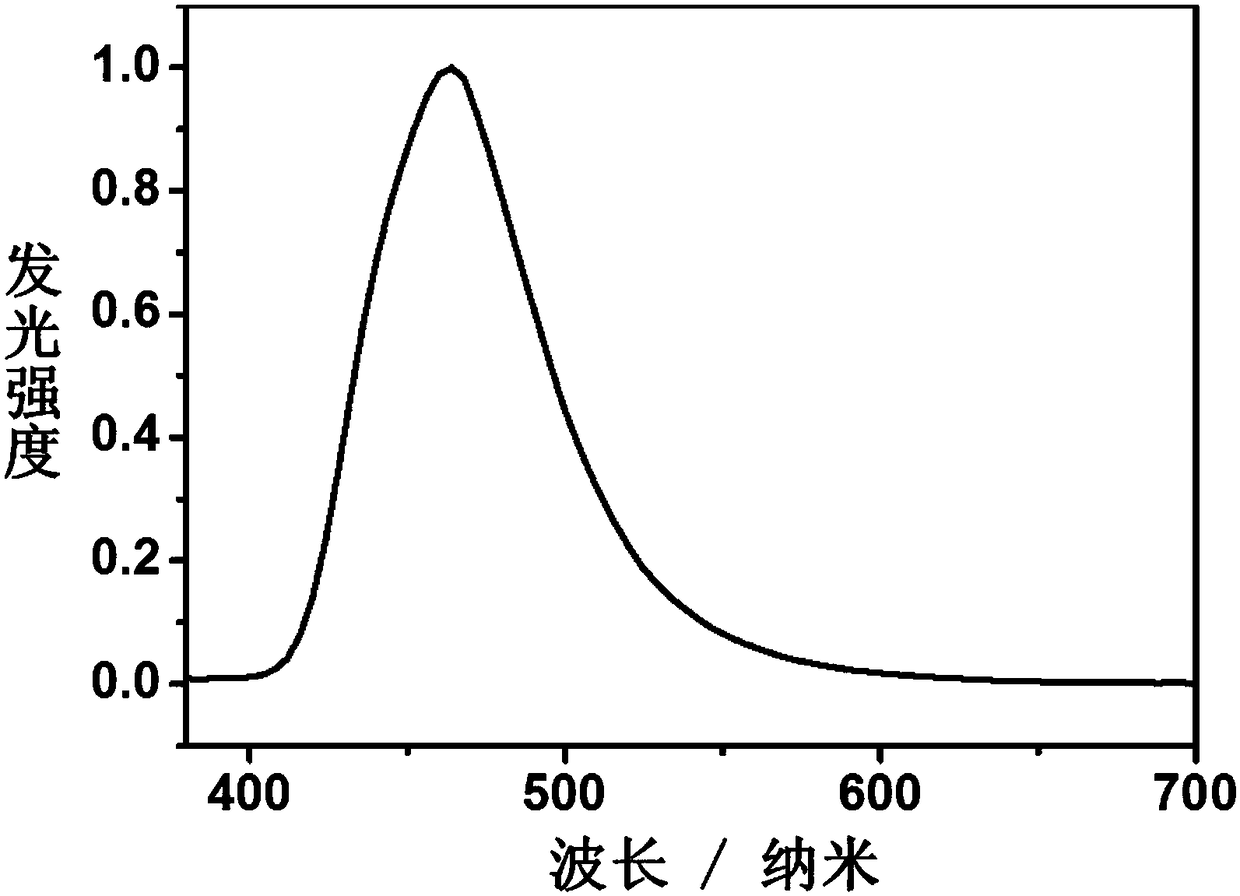
Aggregation-induced luminescent materials of tristyryl-substituted phenanthroimidazole derivatives and their application in the preparation of organic electroluminescent devices
A technology of aggregation-induced luminescence and tristyryl-based, which is applied in luminescent materials, electric solid-state devices, semiconductor devices, etc., and can solve the problems of high device driving voltage, no practical use, and difficulty in growing single crystals.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthesis of compound 1:
[0028]
[0029] Add phenanthrenequinone (12mmol), p-bromobenzaldehyde (12mmol), aniline (32mmol), ammonium acetate (62.5mmol), acetic acid (50mL) into the there-necked flask, N 2 Under protection, it was heated to reflux in an oil bath at 123°C for 12h. The reaction was stopped, the reaction mixture was poured into distilled water, stirred and filtered, the gray filter cake obtained was washed with water, glacial acetic acid and ethanol successively, and after drying, the phenanthroimidazole intermediate (4.57g, yield 85%) obtained by para-bromine substituted . The para-bromo-substituted phenanthroimidazole obtained above (0.90g, 2mmol), 2-(4-(2,2-distyryl)phenyl-4,4,5,5-tetramethyl-1, 3,2-dioxaborolane (0.84g, 2.2mmol), potassium carbonate (1.66g, 12mmol), tetrakis (triphenylphosphine) palladium (23.1mg, 0.02mmol) mixed with 20ml of toluene and 6ml of water were added In a round bottom flask. After degassing three time...
Embodiment 2
[0030] Embodiment 2: the synthesis of compound 2:
[0031]
[0032] According to the synthesis of compound 1, the steps are the same, and p-methylaniline is used instead of aniline to obtain white powder compound 2 (1.05g, yield 82%). The molecular ion mass determined by mass spectrometry is: 638.18 (calculated value: 638.27) ; Theoretical element content (%) C 48 h 34 N 2 : C, 90.25; H, 5.36; N, 4.39; Measured element content (%): C, 90.10; H, 5.33; N, 4.56. The above analysis results indicated that the obtained product was the expected product.
Embodiment 3
[0033] Embodiment 3: the synthesis of compound 3:
[0034]
[0035] According to the synthesis of compound 1, the steps are the same, and aniline is replaced by p-tert-butylaniline to obtain white powder compound 3 (1.06g, yield 78%), and the molecular ion mass determined by mass spectrometry is: 680.55 (calculated value: 680.32 ); Theoretical element content (%) C 51 h 40 N 2 : C, 89.96; H, 5.92; N, 4.11; Measured element content (%): C, 90.02; H, 5.80; N, 4.17. The above analysis results indicated that the obtained product was the expected product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com