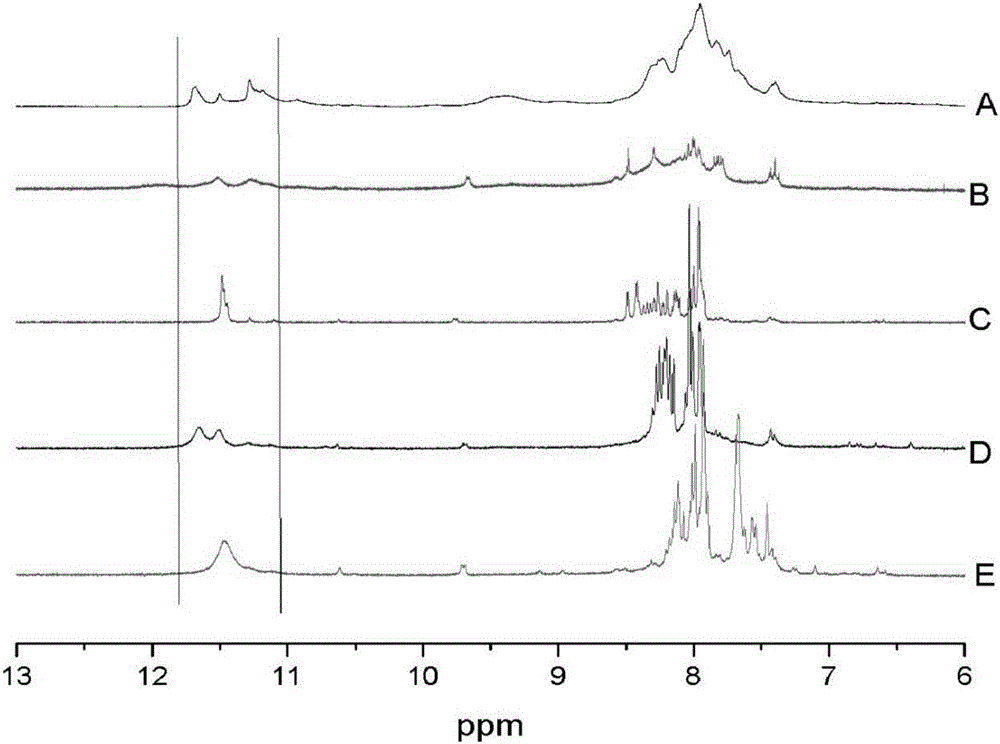
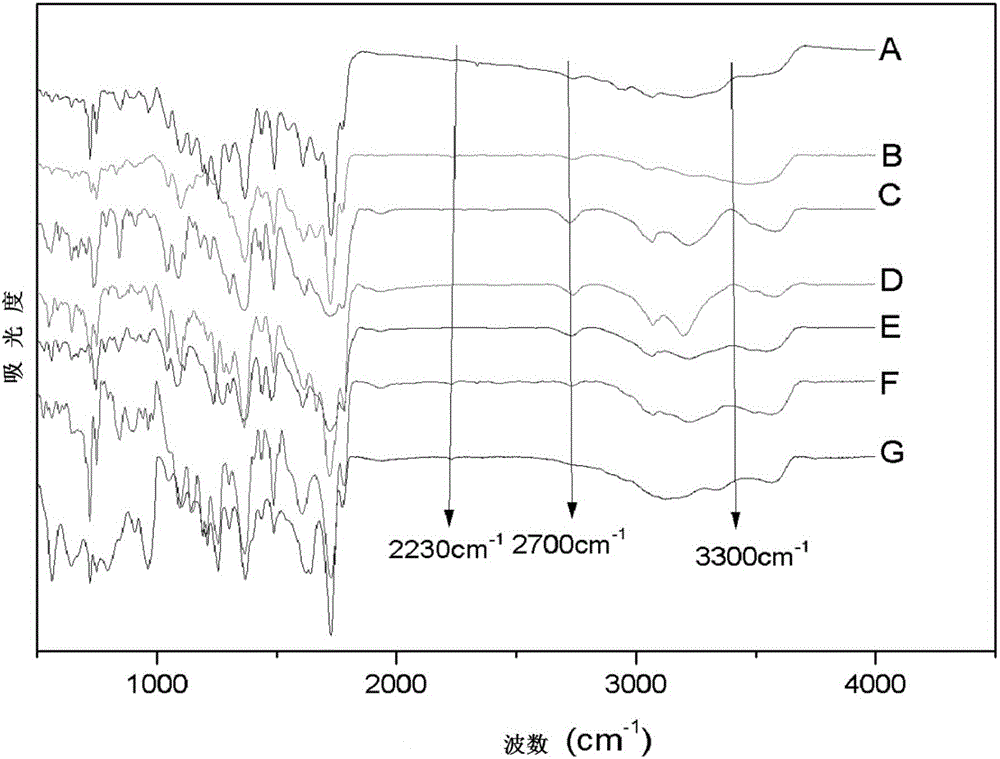
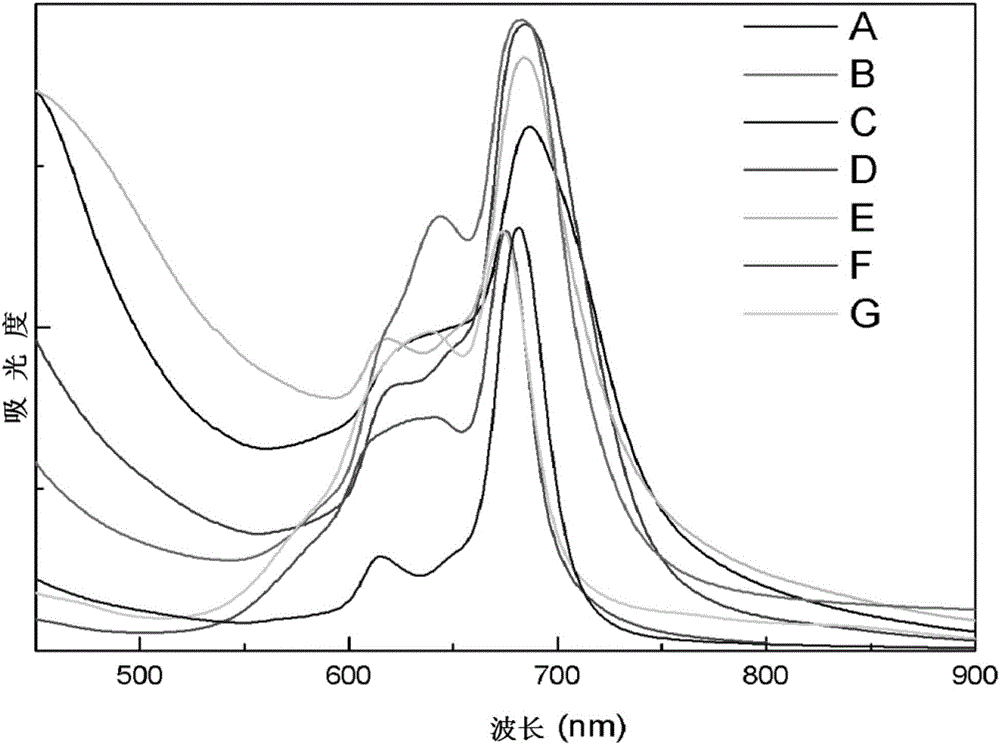
Hyperbranched metal phthalocyanine oligomer containing imide structure and preparation method thereof
A technology containing imide and metal phthalocyanine is applied in the field of hyperbranched metal phthalocyanine oligomer and its preparation, and can solve the problems of reducing thermal decomposition temperature and conjugation degree of phthalocyanine polymer, adverse effects on material properties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0033] Embodiment 1~5 (preparation of tetracyano-terminated monomer containing imide structure)
[0034] 4-Aminophthalonitrile was dissolved in dry dimethyl sulfoxide (DMSO) solvent and placed in a container, and the corresponding anhydride monomers (A, B, C, D and E), after rapid stirring for half an hour, add a mixed solution of acetic anhydride and pyridine (volume ratio 5:4) to the reaction system, raise the temperature of the reaction system to 60°C, and react for 10 hours. After the reaction, cool to room temperature and discharge into distilled water, wash repeatedly with deionized water, recrystallize with acetonitrile, and dry in an oven to obtain the corresponding tetracyano-terminated monomer containing imide structure. The corresponding relationship between the types of acid anhydride monomers and products (tetracyano-terminated monomers containing imide structures) is shown in Table 1.
[0035] Table 1
[0036]
[0037]
Embodiment 6~12
[0038] Embodiment 6~12 (preparation of hyperbranched zinc phthalocyanine oligomer containing imide structure)
[0039]In the three-necked bottle, the three-necked bottle is sequentially equipped with a nitrogen vent, a glass stopper and a drying tube. Dissolve 3mmol of the corresponding tetracyano-terminated monomer (a, b, c, d or e) containing imide structure in 40mL of N,N-dimethylacetamide (DMAc), add 1mmol of transition metal salt , adding the catalyst, heating to 160°C under nitrogen atmosphere for continuous reaction for 24h. After the reaction was cooled to room temperature, the reaction mixture was poured into 600 mL of deionized water with a pH<1. After the crude product was collected by filtration, it was washed with deionized water and acetonitrile respectively under reflux to remove the remaining transition metal salts and tetracyano-terminated monomers containing imide structures. After washing until the filtrate was colorless, the product was rinsed with cold et...
Embodiment 13
[0043] The difference between this example and examples 6-12 is that the solvent used is dimethylformamide (DMF), N-methylpyrrolidone (NMP) or quinoline. Heating to 150-160° C. under nitrogen protection environment for continuous reaction for 20-24 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com