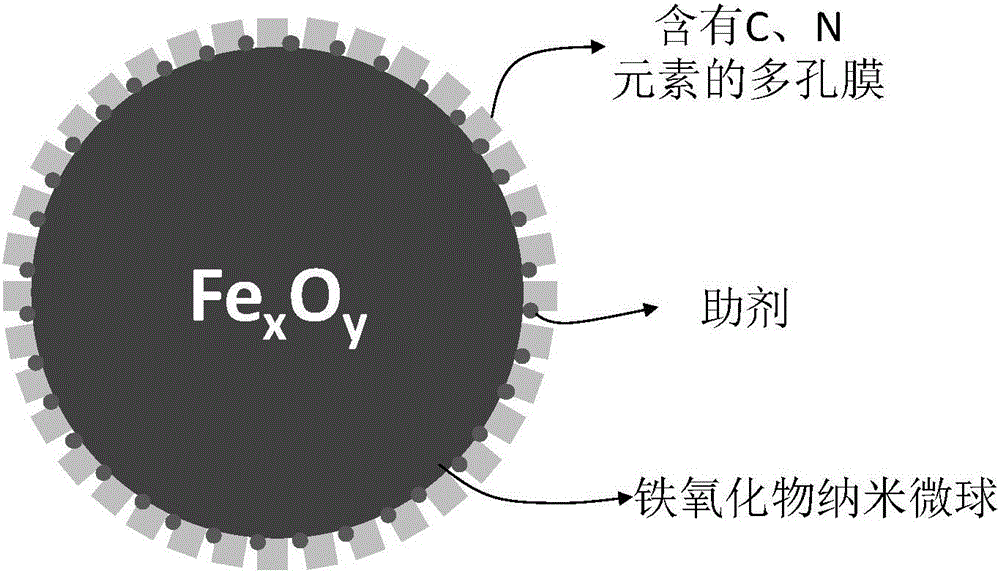
Application of iron-based catalyst with porous film structure in Fischer-Tropsch reaction
A Fischer-Tropsch reaction and catalyst technology, applied in the field of preparation of iron-based catalysts, can solve the problem of no application of iron nanoparticles, and achieve the effects of preventing carbon deposition and sintering, and inhibiting deactivation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
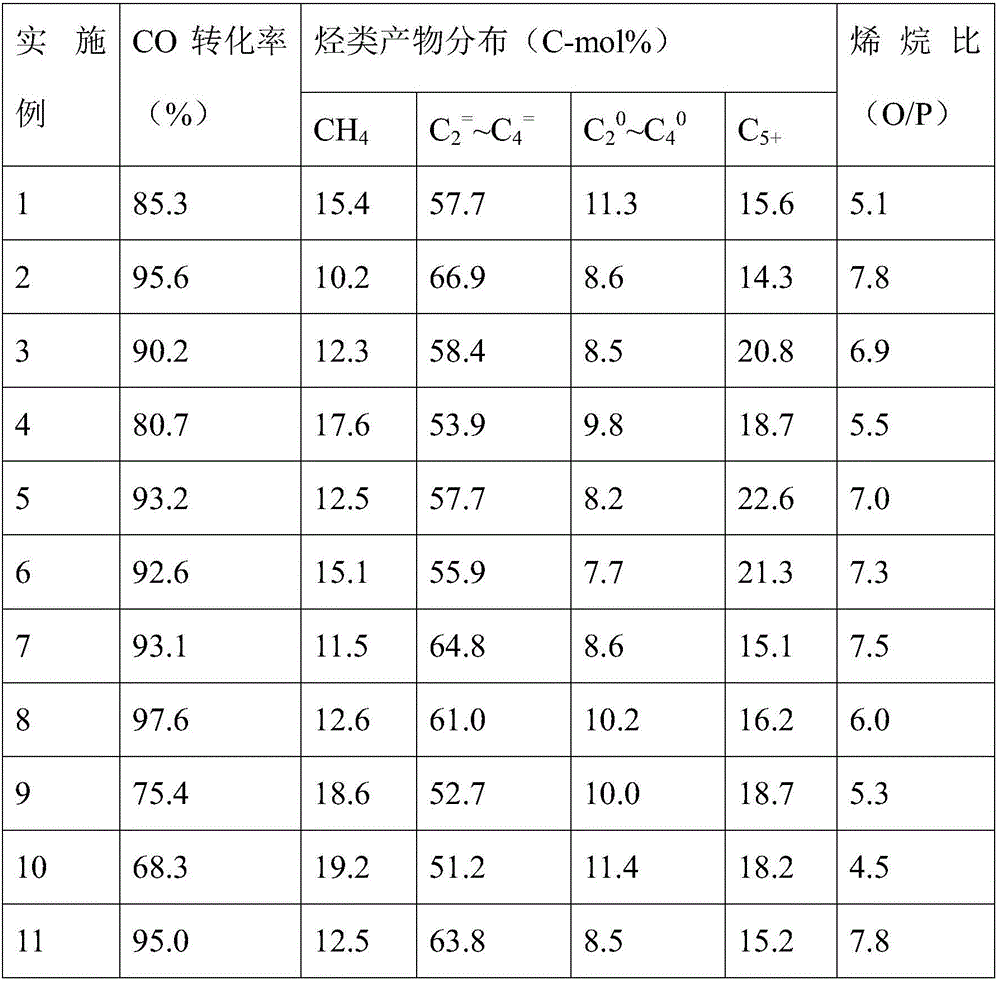
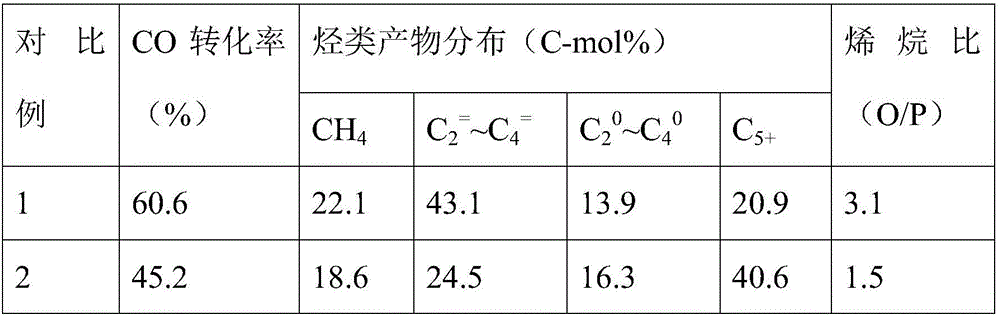
Examples
Embodiment 1
[0023] 1) Fe 2 o 3 Preparation of Nanospheres. In a 500mL three-necked bottle, add 3.240g FeCl 3 ·6H 2 O and 300mL deionized water, reflux at 100°C for 80h. After cooling to room temperature, centrifuge and wash with deionized water until no chloride ions exist in the filtrate. And continue to wash with absolute ethanol for 3 times, then vacuum dry at room temperature for 12h to obtain Fe 2 o 3 nanospheres.
[0024] 2) Coating the nano-microspheres in step (1) with polyacrylic acid. Take 0.5gFe 2 o 3 Nanospheres were dispersed in 100mL deionized water, and 0.022g KNO was added 3 1. 5g of polyacrylic acid, ultrasonic for 30min, stirred and impregnated at room temperature for 5h, then evaporated under negative pressure at 80°C to dryness, and dried at 120°C for 12h. Finally, under the protection of nitrogen, it was calcined at 450 °C for 4 h in a tube furnace to obtain carbon film-coated Fe 2 o 3 catalyst.
[0025] 3) Activity test. The activity of the prepared ca...
Embodiment 2
[0027] 1) Fe 2 o 3 Preparation of Nanospheres. In a 500mL three-necked bottle, add 3.240g FeCl 3 ·6H 2O and 300mL deionized water, reflux at 100°C for 40h. After cooling to room temperature, centrifuge and wash with deionized water until no chloride ions exist in the filtrate. And continue to wash with absolute ethanol for 3 times, then vacuum dry at room temperature for 12h to obtain Fe 2 o 3 nanospheres.
[0028] 2) Coating the nanospheres in step (1) with polyvinylpyrrolidone. Take 0.5gFe 2 o 3 Nanospheres were dispersed in 100mL deionized water, and 0.015g CH 3 COOK, 5g polyvinylpyrrolidone, sonication for 30min, stirring and impregnating at room temperature for 5h, then vacuum rotary evaporation at 80°C to dryness, and drying at 120°C for 12h. Finally, under the protection of nitrogen, it was roasted in a tube furnace at 250°C for 2 hours to obtain Fe coated with carbon and nitrogen. 2 o 3 catalyst.
[0029] 3) The activity test conditions are the same as in...
Embodiment 3
[0031] 1) Fe 2 o 3 Preparation of Nanospheres. In a 500mL three-necked bottle, add 2.592g FeCl 3 ·6H 2 O and 300mL deionized water, reflux at 90°C for 90h. After cooling to room temperature, centrifuge and wash with deionized water until no chloride ions exist in the filtrate. And continue to wash with absolute ethanol for 3 times, then vacuum dry at room temperature for 12h to obtain Fe 2 o 3 nanospheres.
[0032] 2) Coating the nanospheres in step (1) with polyethyleneimine. Take 0.5gFe 2 o 3 Nanospheres were dispersed in 100mL deionized water, and 0.018gK was added 2 CO 3 1. 5g of polyethyleneimine, ultrasonic for 30min, stirred and impregnated at room temperature for 5h, then evaporated under negative pressure at 80°C to dryness, and dried at 120°C for 12h. Finally, under the protection of nitrogen, it was roasted in a tube furnace at 200 °C for 1 h to obtain Fe coated with two elements of carbon and nitrogen. 2 o 3 catalyst.
[0033] 3) The activity test co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com