Low-viscosity star-shaped hydroxyl polyester as well as preparation method and application thereof
A hydroxyl polyester, low-viscosity technology, applied in the field of organic polymer compounds, can solve problems such as complicated operation, long time consumption, and non-compliance with environmental protection requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Raw material composition
[0057]
[0058] 2. Preparation
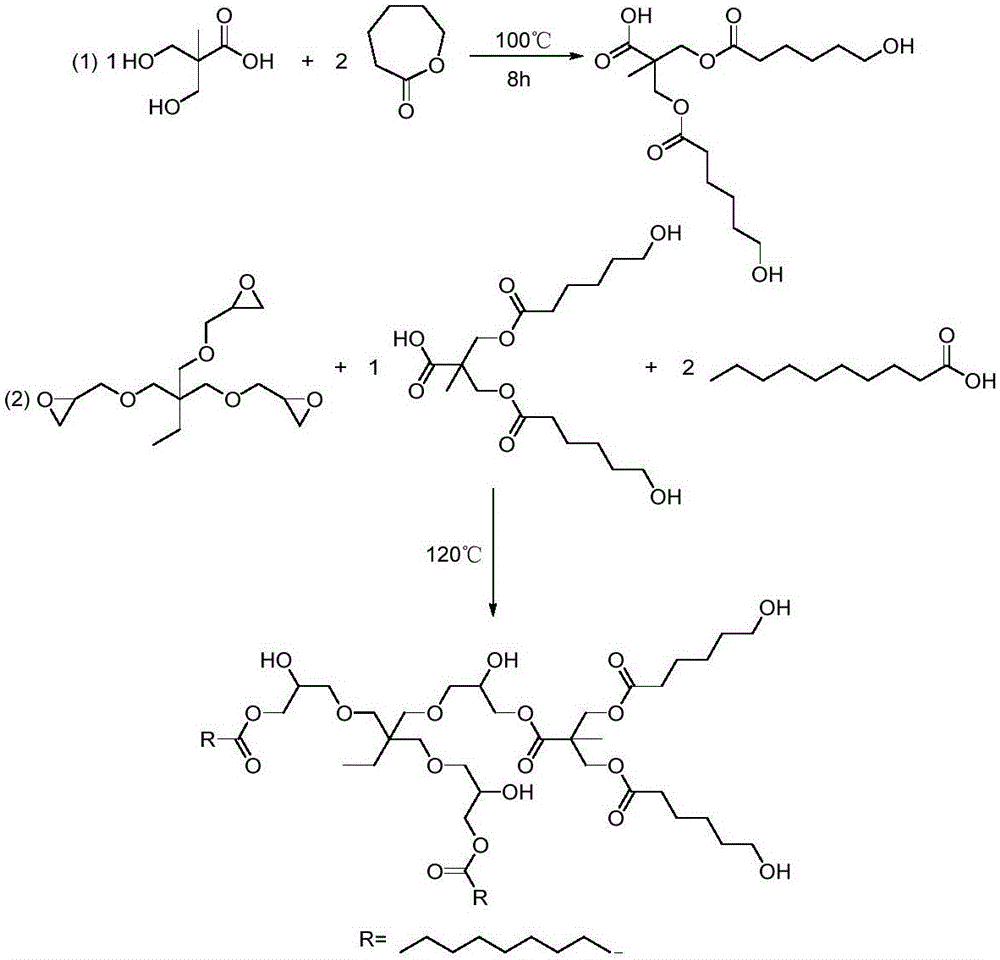
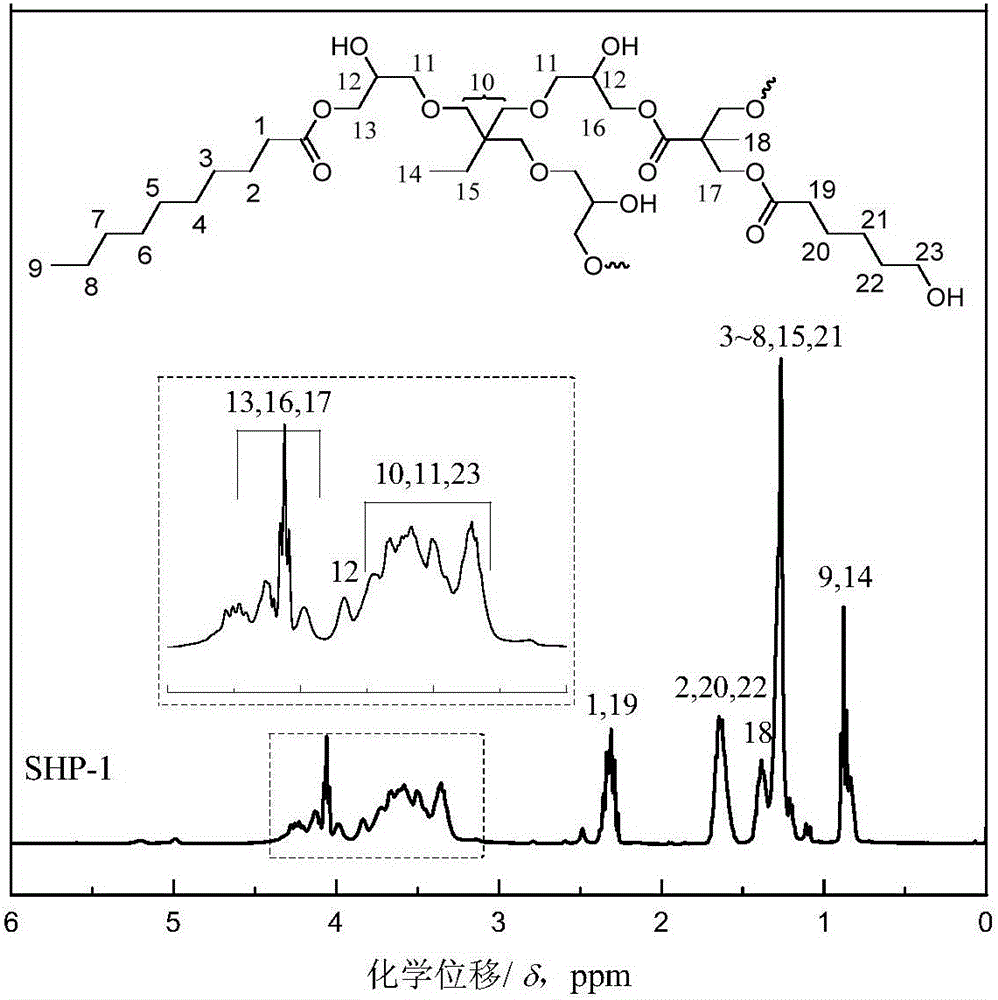
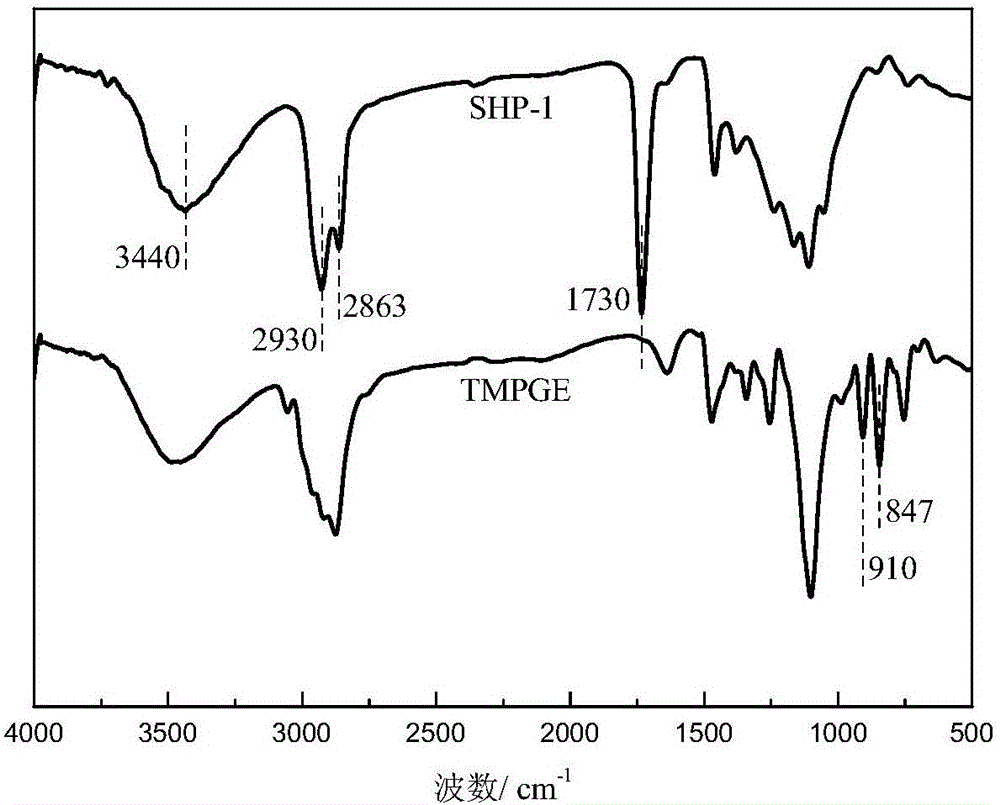
[0059] The synthesis principle of star-shaped hydroxyl polyester SHP-1 is as follows: Picture 1-1 As shown, it specifically includes the following steps:
[0060] (a) Caprolactone modified AB 2 Type branched monomer: In a four-necked flask equipped with a mechanical stirrer, a thermometer, a spherical condenser, and a nitrogen port, add 134.1g dimethylolpropionic acid, 228.3g caprolactone, and 1.0g anhydrous zinc acetate , reacted at 100°C for 6 hours under the protection of nitrogen, the infrared characteristic peak of caprolactone in the reaction system detected by infrared spectroscopy disappeared, and the solid content of the detected reaction system was >99%, and the AB modified by caprolactone was obtained by cooling down. 2 type branched monomer.
[0061] (b) Preparation of low-viscosity star-shaped hydroxyl polyester: modified AB prepared in step (a) 2 Add 422.5g trimethylolpropane triglycidy...
Embodiment 2
[0072] 1. Raw material composition
[0073]
[0074] 2. Preparation
[0075] The synthesis principle of star-shaped hydroxyl polyester SHP-2 is as follows: figure 2 As shown, it specifically includes the following steps:
[0076] (a) Caprolactone modified AB 2 Preparation of type branched monomer: In a four-necked flask equipped with a mechanical stirrer, a thermometer, a spherical condenser, and a nitrogen port, add 134.1g dimethylolpropionic acid, 114.1g caprolactone, and 0.25g dilaurel Dibutyltin acid, reacted at 140°C for 5 hours under the protection of nitrogen, the infrared characteristic peak of caprolactone in the reaction system detected by infrared spectroscopy disappeared, and the solid content of the detected reaction system was >99%, and the AB modified by caprolactone was obtained by cooling down. 2 type branched monomer.
[0077] (b) Preparation of low-viscosity star-shaped hydroxyl polyester: add 422.5g trimethylolpropane triglycidyl ether, 565.0g oleic...
Embodiment 3
[0084] 1. Raw material composition
[0085]
[0086] 2. Preparation
[0087] The synthesis principle of star-shaped hydroxyl polyester SHP-3 is as follows: image 3 As shown, it specifically includes the following steps:
[0088] (a) Caprolactone modified AB 2 Preparation of type branched monomer: In a four-necked flask equipped with a mechanical stirrer, a thermometer, a spherical condenser, and a nitrogen port, add 134.1g dimethylolpropionic acid, 456.6g caprolactone, 1.6g anhydrous Zinc acetate was reacted at 120°C for 6 hours under the protection of nitrogen. The infrared characteristic peak of caprolactone in the reaction system detected by infrared spectroscopy disappeared, and the solid content of the reaction system was detected to be >99%. The AB modified by caprolactone was obtained by cooling down. 2 type branched monomer.
[0089] (b) Preparation of low-viscosity star-shaped hydroxyl polyester: add 250g resorcinol diglycidyl ether, 282.5g oleic acid, 1.6g te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com