Red to near-infrared phosphorescent iridium complex light-emitting material and application thereof to electroluminescent device
A technology for phosphorescent iridium complexes and luminescent materials, which is applied in the field of red to near-infrared phosphorescent iridium complex luminescent materials, and can solve problems such as high prices, difficult industrial production, and difficulties in large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
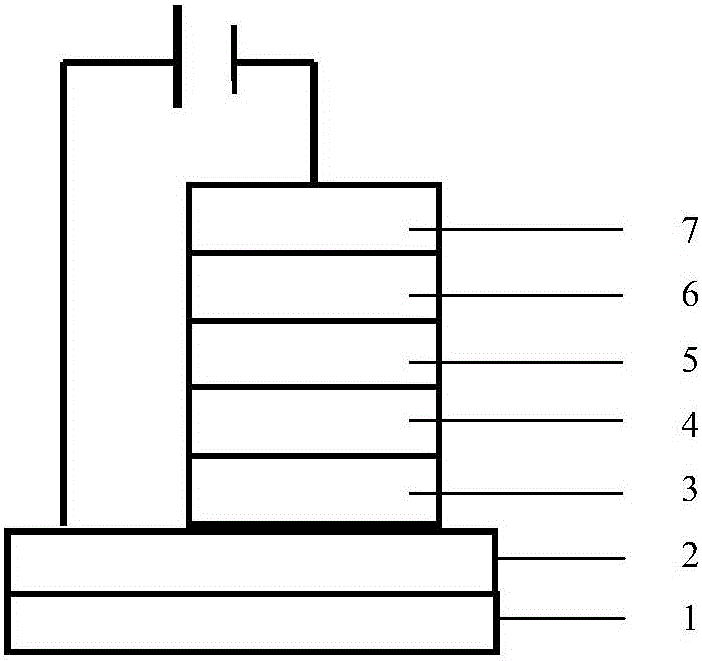
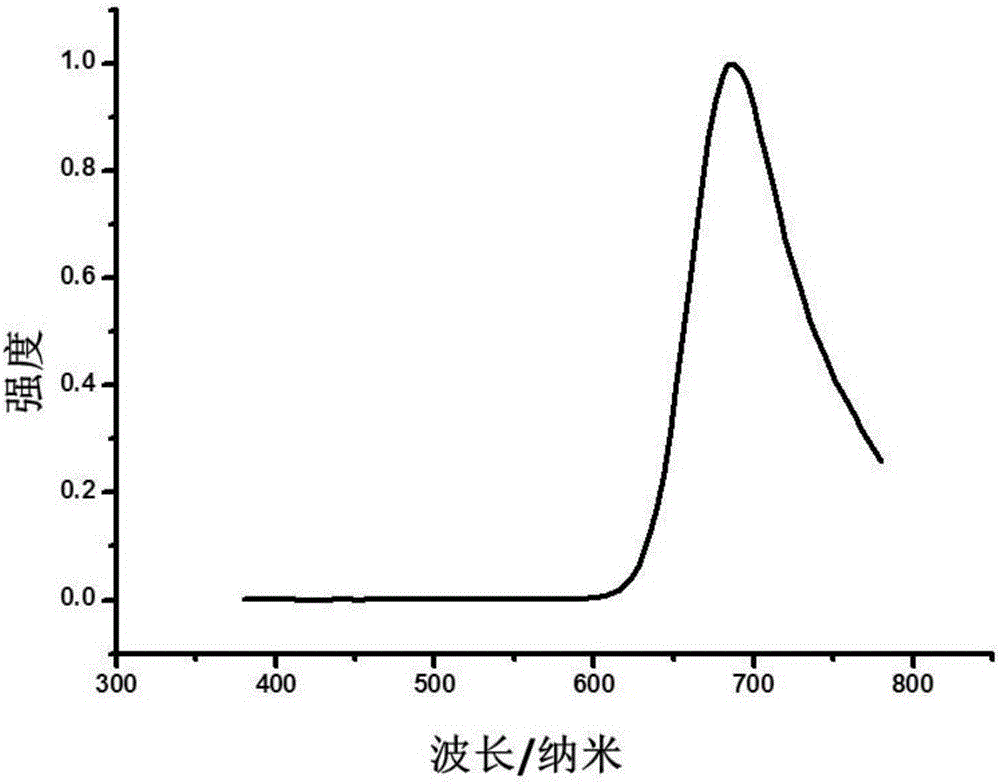
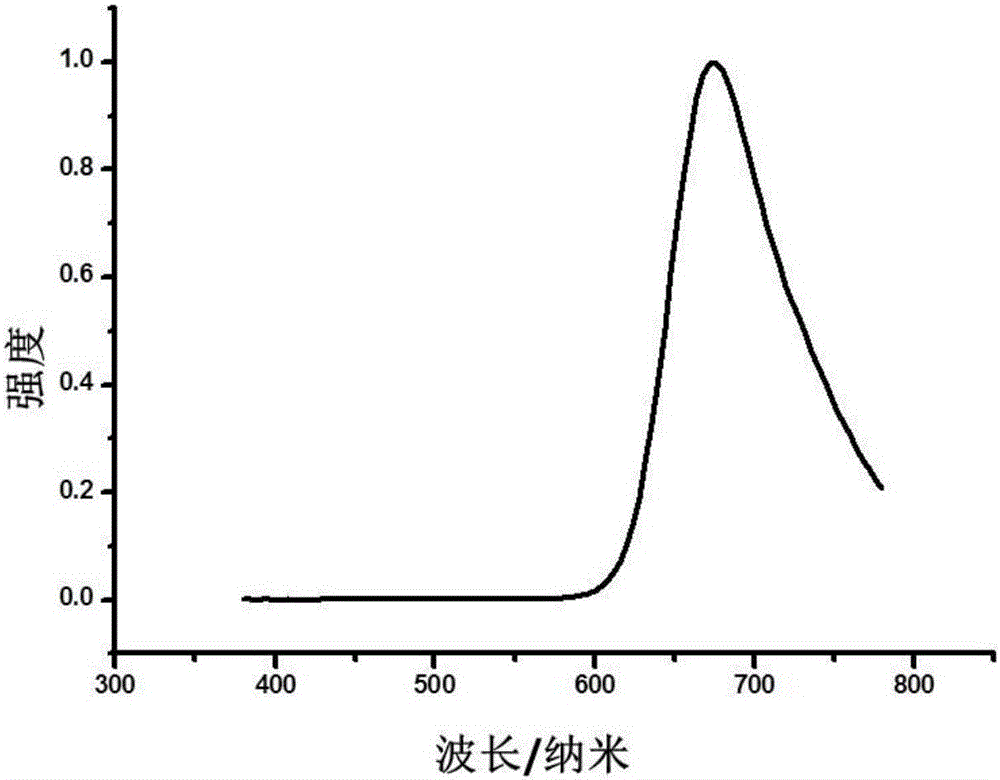
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of compound 1-phenylisoquinoline (PIQ):
[0041]
[0042] Add phenylboronic acid (16mmol), 1-chloroisoquinoline (12mmol), tetrakis(triphenylphosphine)palladium (0.06mmol), 2mol / L sodium carbonate solution (60ml), tetrahydrofuran (60mL) into a two-necked flask , N 2 Under protection, heat and reflux in an oil bath at 123°C for 12 hours. Stop the reaction, pour the reaction mixture into distilled water, extract and separate the liquids with dichloromethane, collect the organic phase, and then separate it by column chromatography (silica gel, dichloromethane) The target product was obtained as white powder.
[0043] The synthesis method of other 1-phenylisoquinoline derivatives containing various substituent groups involved in the present invention is the same, and can be synthesized from phenylboronic acid substituted by corresponding substituents according to the above reaction conditions.
Embodiment 2
[0044] Embodiment 2: Chlorine bridged intermediate [Ir(piq) 2 (μ-Cl)] 2 Synthesis:
[0045]
[0046] 1-phenylisoquinoline (12mmol), iridium trichloride trihydrate (5mmol), ethylene glycol ether (15ml), purified water (5ml) were added in the two-necked flask, N 2 Under protection, it was heated to reflux in an oil bath at 150°C for 12h. Stop the reaction, add 150 mL of distilled water to precipitate a precipitate, filter the reaction mixture, wash the filter cake with absolute ethanol, and dry to obtain the red powdery target product.
[0047] The synthesis method of other iridium chloride bridged intermediate derivatives containing various substituent groups involved in the present invention is the same, and can be synthesized from corresponding 1-phenylisoquinoline derivatives according to the above reaction conditions.
Embodiment 3
[0048] Embodiment 3: the synthesis of compound 1:
[0049]
[0050] Add 41mg (0.4mmol) of diisopropylamine and 10mL of n-hexane into a 50mL three-necked flask, under nitrogen protection and at -78°C, add 0.15mL of 2.6M / L n-butyllithium dropwise, react for one hour under stirring, and add dropwise N , N′-diisopropylcarbodiimide 50mg (0.4mmol), after the dropwise addition, gradually rise to room temperature, continue to react for one hour under stirring, slowly add 0.2mmol of [Ir( piq) 2 (μ-Cl)] 2 in n-hexane solution. After the dropwise addition was completed, the temperature was slowly raised to 80° C., and the reaction was carried out for 8 hours. The reaction was stopped, the mixture was cooled to room temperature, the solvent was spin-dried under reduced pressure, and the obtained solid product was washed three times with ether, 20 mL each time. Vacuum sublimation obtained red powdery compound 1 (281.2 mg, yield 85%), and the molecular ion mass determined by mass spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com