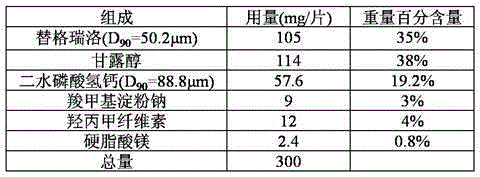
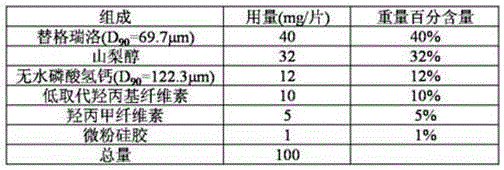
Tablet containing ticagrelor
A technology for ticagrelor and tablets, applied in the field of tablets containing ticagrelor and its preparation, can solve the problems of drug loss, unfavorable production operations, high production costs, etc., achieve high bioavailability and facilitate absorption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107]
[0108] First mix hypromellose and low-substituted hydroxypropyl cellulose evenly, and then mix with the same amount of ticagrelor, so that the amount is increased until all the ticagrelor is mixed, and then mixed with anhydrous hydrogen phosphate Calcium is mixed evenly, and finally sorbitol is added, mixed evenly, granulated with aqueous solution through a 24-mesh sieve, dried at 60°C, granulated, added with micropowder silica gel, mixed evenly, compressed into tablets, and coated.
Embodiment 2
[0110]
[0111] First mix the hypromellose and crospovidone evenly, then mix with the same amount of ticagrelor, so that the amount is increased until all the ticagrelor is mixed, and then mixed with anhydrous calcium hydrogen phosphate Uniformly, add lactose at the end, mix evenly, sieve with 24 mesh, wet granulate with aqueous solution, dry at 70°C, granulate, add talcum powder, mix evenly, compress into tablets, and coat.
Embodiment 3
[0113]
[0114] First mix hypromellose with hydroxypropyl cellulose and croscarmellose sodium evenly, then mix with the same amount of ticagrelor, and increase the amount until all ticagrelor is mixed. , mixed evenly with sucrose, then mixed evenly with calcium orthophosphate, finally mixed evenly with mannitol, granulated with an aqueous solution through a 24-mesh sieve, dried at 70°C, granulated, added with talc powder and magnesium stearate, mixed evenly After compression, coating.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com