Alpha crystal form of obeticholic acid as well as preparation method, medicine composition and application thereof
A technology of obeticholic acid and crystal form, which is applied in the field of α crystal form of obeticholic acid and its preparation, can solve problems such as low melting temperature, unsuitability, and potential safety hazards, and achieve simple process operation and good stability , low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] Preparation of α-crystal form of obeticholic acid
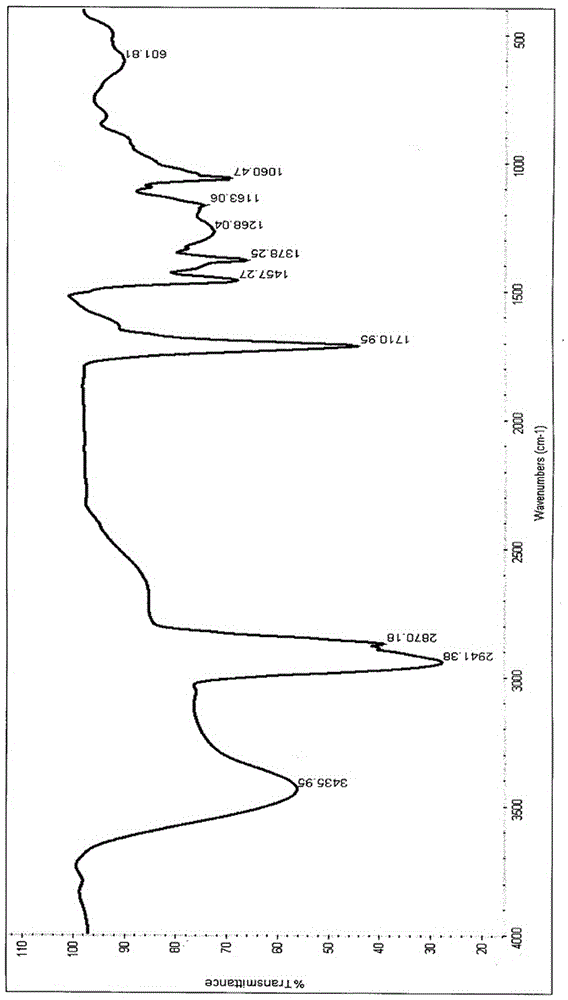
[0065] Testing instruments and methods:
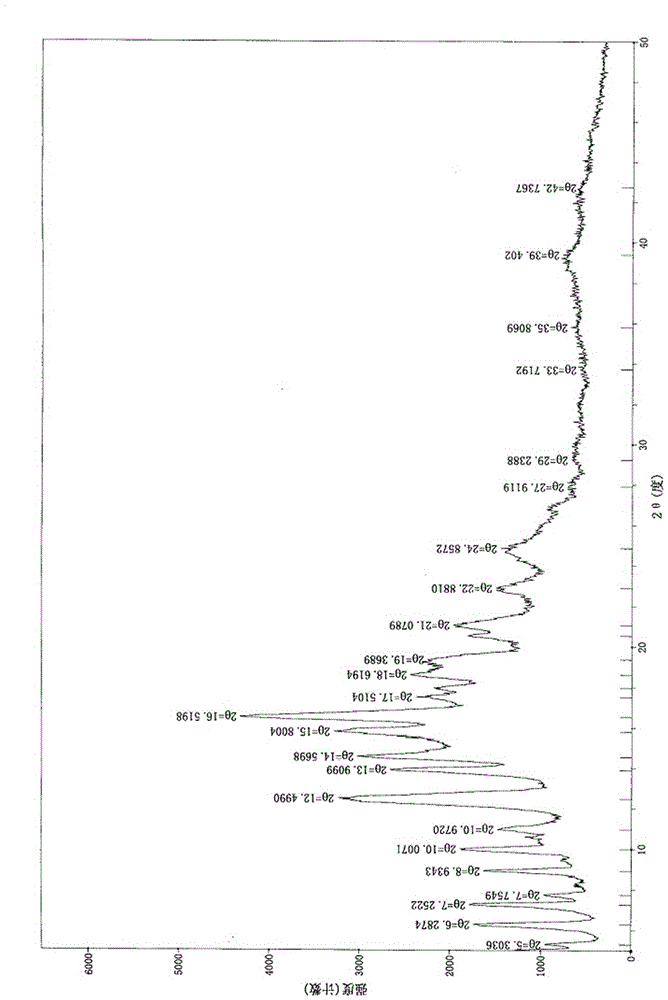
[0066] X-ray powder diffraction (XRPD)
[0067] Instrument: DX-2700B X-ray diffractometer
[0068] Sample preparation: Use the original sample for detection, that is, without grinding the sample, directly take about 50 mg of sample and gently flatten it on a glass slide to obtain a flat surface.
[0069] Sample determination: put the sample into a sample holder for X-ray powder diffraction determination, use a DX-2700B X-ray diffractometer equipped with Cu-Kα radiation (α=1.54059nm), and the effective 2θ range is 5° -50°, the step angle is 0.03 seconds (0.01-0.03), the software used to collect data is X-ray diffractometer 2.1 software, and MDJJade9.0 analysis software is used to present the data.
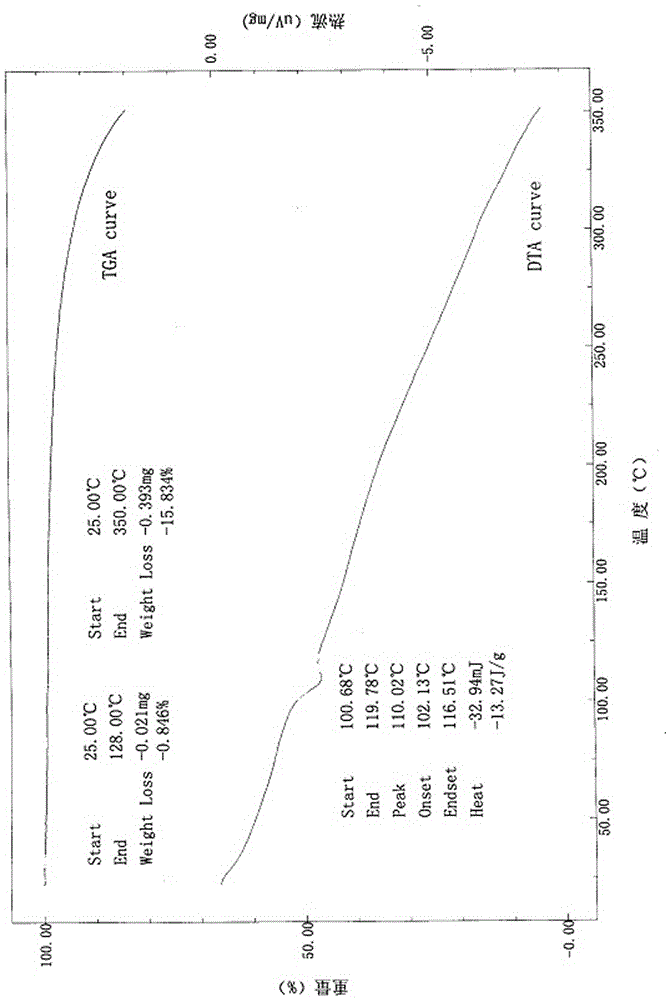
[0070] Differential thermal-thermogravimetric analysis
[0071] Equipment: Japan Shimadzu DTG-60(H)
[0072] TGA (Thermogravimetric Analysis) data was collected on a Mettler TGA...
Embodiment 1
[0076] Add 10g of obeticholic acid and 120ml of toluene into a three-necked reaction flask with a reflux condenser, heat to reflux with stirring, stop heating after obeticholic acid is completely dissolved, and lower the crystallization system to 60°C within half an hour while stirring. ℃. After the crystallization system was stably lowered to 60° C., the crystallization system was lowered to room temperature within half an hour under stirring. Then, the temperature of the crystallization system was lowered to 0° C. under stirring, and the crystallization was stirred at 0° C. for 12 hours. After filtering and washing with 5 ml of toluene, the obtained crystals were placed in an oven at 70° C. and dried under normal pressure for 12 hours to obtain 9.3 g of fine needle crystals, yield: 93%. mp: 106.0-108.0°C; HPLC purity: 99.83%; water content (Karl Fischer method): 0.92%.
Embodiment 2
[0078] Add 10g of obeticholic acid and 150ml of toluene to a three-neck reaction flask with a reflux condenser, heat to reflux under stirring, after obeticholic acid is completely dissolved, add 0.3g of activated carbon, decolorize under reflux for 30 minutes, filter, Collect the filtrate. The filtrate was transferred to a three-port reaction and continued heating to reflux. Make sure that the solution heated to reflux is completely clear, stop heating and stir. The crystallization system was naturally lowered to room temperature, and after crystallization at room temperature for 6 hours, it was frozen and crystallized at -5°C for 10 hours. After suction filtration and washing with 5 ml of toluene, the obtained crystals were placed in an oven at 60° C., dried under reduced pressure at 150 mmHg for 8 hours, and then 9.0 g of fine needle crystals were obtained. Yield: 90%. mp: 107.0-108.0°C; HPLC purity: 99.66%; water content (Karl Fischer method): 0.78%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com