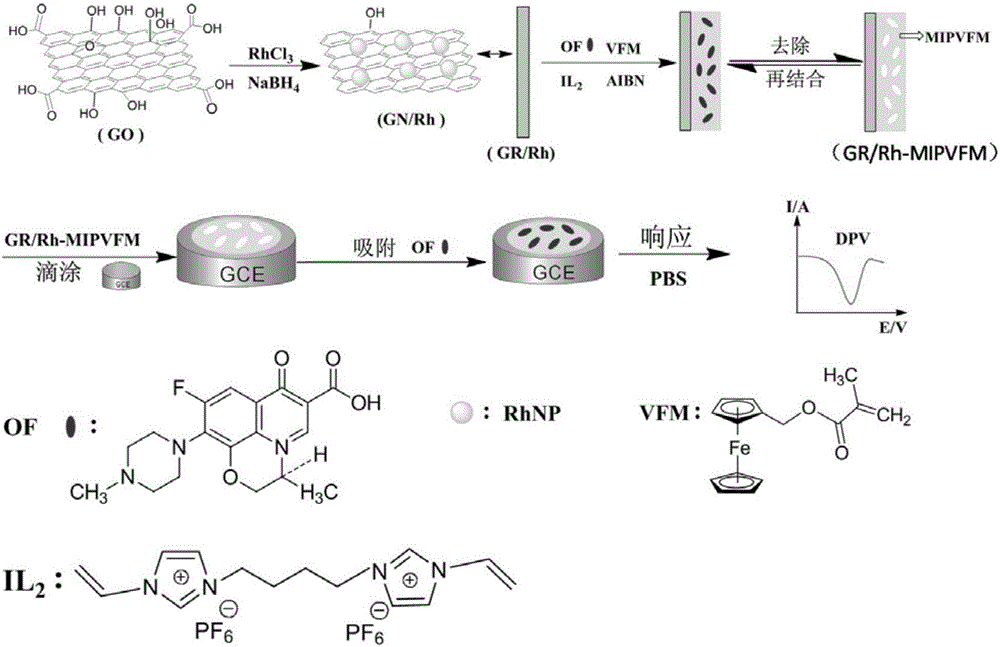
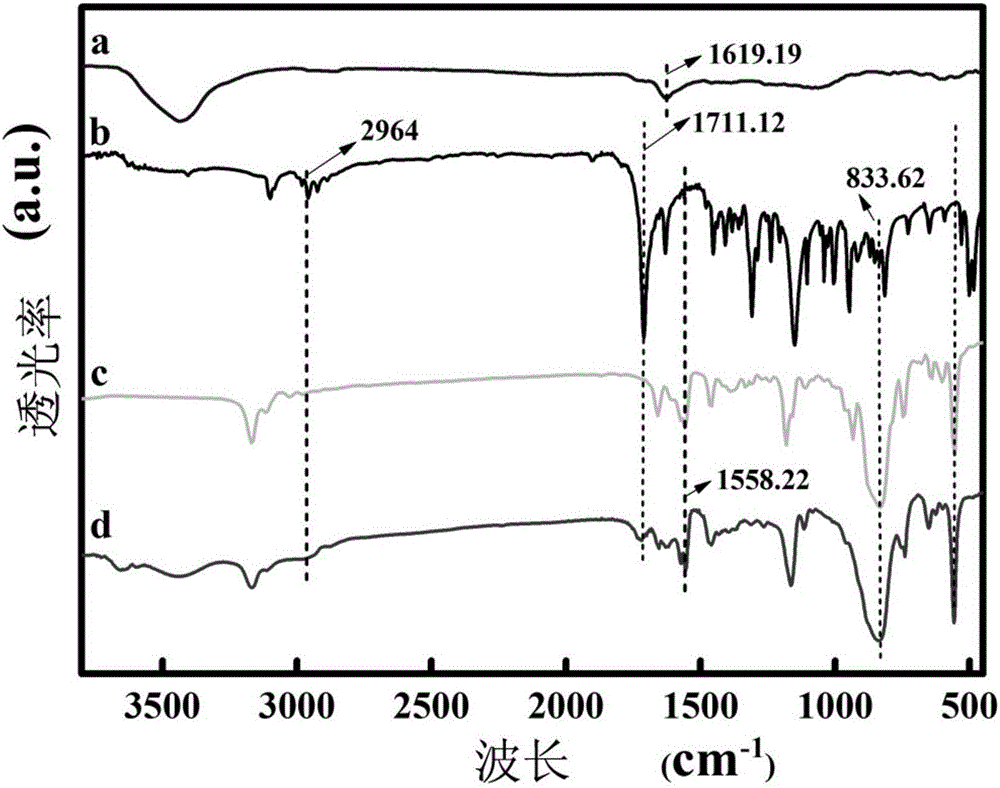
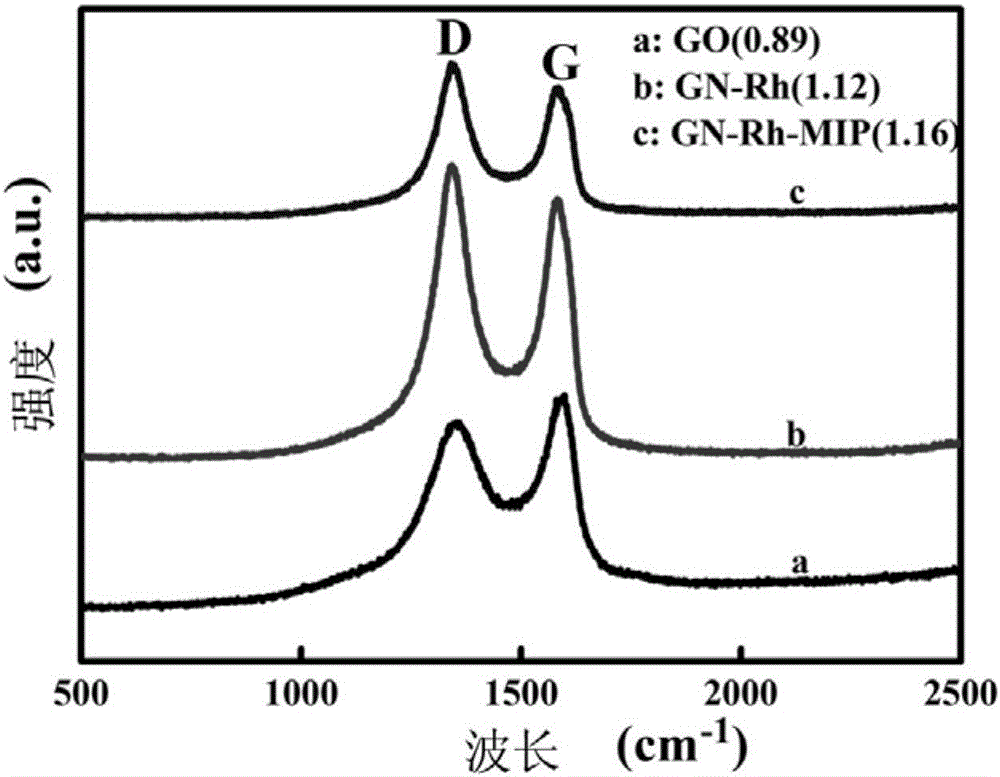
Molecularly imprinted polymer for detecting ofloxacin and preparing method and application thereof
A molecular imprinting, polymer technology, applied in chemical instruments and methods, other chemical processes, material electrochemical variables, etc., can solve problems such as affecting the ecological environment, affecting microbial functions, and improving bacterial resistance, achieving strong selectivity. , Detection of wide linear range, less susceptible to interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 graphene / rhodium nanoparticle preparation
[0040] Dissolve 20mg of graphene oxide (GO) in 20mL of ethanol, and disperse it evenly by ultrasonication for 1h; then add 400μL of 0.1M RhCl 3 solution and 4.0 x 10 -4 mol of sodium borohydride, ultrasonication for two hours; finally, the solution was condensed and refluxed at 90°C for 12 hours to obtain a black suspension solution, which was washed by centrifugation and dried overnight in a vacuum oven to obtain graphene / rhodium nanoparticles (GN / Rh).
Embodiment 2
[0041] Embodiment 2 ionic liquid preparation
[0042] (1) 3,3'-(1',4'-butane)-bis-1-vinylimidazolium bromide ([C 4 (mim) 2 ][Br 2 ])Synthesis
[0043] Mix 0.1mol 1-vinylimidazole and 0.05mol 1,4-dibromobutane evenly, add 30mL methanol, stir the mixture at 60°C for 15h to obtain a light yellow viscous liquid, cool to room temperature after the reaction is complete . Then the viscous solution was poured into 500mL anhydrous ether for washing, and the lower layer of viscous solution was decanted gently and vacuum-dried overnight at room temperature to constant weight to obtain [C 4 (mim) 2 ][Br 2 ]. NMR characterization obtained [C 4 (mim) 2 ][Br 2 ], the result is as follows: 1HNMR (400MHz, DMSO-d 6 ,δ,ppm):9.54s,2H),8.28(s,2H),8.02(s,2H),7.40dd,2H),6.00(d,2H),5.41(d,2H),4.29(s, 4H), 1.87(s, 4H); 13C NMR ((400MHz, DMSO-d 6 , δ, ppm): 135.66, 129.28, 123.71, 119.19, 109.06, 48.39, 25.75.
[0044] The specific reaction formula is:
[0045]
[0046] (2) 3,3′-(1′,4...
Embodiment 3
[0050] Example 3 Preparation of Molecularly Imprinted Polymers of the Present Invention
[0051] First, 0.080g carrier GN / Rh, 0.1mmol template molecule ofloxacin and 0.4mmol functional monomer ferrocenylmethyl methacrylate (VFM) were dispersed in 30mL of acetonitrile and toluene mixed solution (V: V=1:1), after ultrasonic mixing, 2.0mmol cross-linking agent [C 4 (mim) 2 ][(PF 6 ) 2 ] and 20mg of initiator azobisisobutylcyanide were added to the mixed solution, and nitrogen gas was introduced for 15 minutes, and then the reaction vessel was sealed. Then, it was reacted in an oil bath at 60°C for 24h, and the obtained solid product was dried in a vacuum oven at 50°C. Finally, Soxhlet extraction was carried out with a Soxhlet extractor, and the mixed solution of methanol and acetic acid (V:V=9:1) eluted the template molecules to obtain the molecularly imprinted polymer GN / Rh-MIP. At the same time, the molecularly imprinted polymer (GN / Rh-MIPMAA) with methacrylic acid as the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com