A kind of hyperbranched polytriazole functionalized graphene and preparation method thereof
A technology of polytriazole and functionalization, which is applied in the field of hyperbranched polytriazole functionalized graphene and its preparation, can solve problems such as the destruction of graphene lattice structure, achieve excellent physical and chemical properties, ensure lattice structure, excellent The effect of mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] Correspondingly, the preparation method of hyperbranched polytriazole functionalized graphene in the present invention includes the following steps:
[0052] (1) Dissolve 3,5-dihydroxybenzoic acid in methanol, and obtain the reaction product under the catalysis of hydrochloric acid (before the reaction system reacts, the concentration of 3,5-dihydroxybenzoic acid is 50.1-66.6mg / mL, hydrochloric acid The concentration is 6.2~8.1mg / mL), the reaction temperature is 90~100℃, and the reaction time is 12~24 hours. After separation and purification, the reaction product compound A is obtained (the main process of separation and purification can be: The product compound A is recrystallized with deionized water; the choice of solvent during the recrystallization process ensures that compound A can be dissolved in the solvent, while other impurities, such as unreacted raw materials, cannot be used, so that the reaction product compound A) can be isolated.
[0053] (2) Dissolve compoun...
Embodiment 1
[0061] Hyperbranched polytriazole functionalized graphene, the end of the hyperbranched polytriazole is grafted with aromatic groups, and then uniformly modified to the surface of the graphene through non-covalent bonding. The aromatic group is The non-covalent bond function is π-π conjugation.
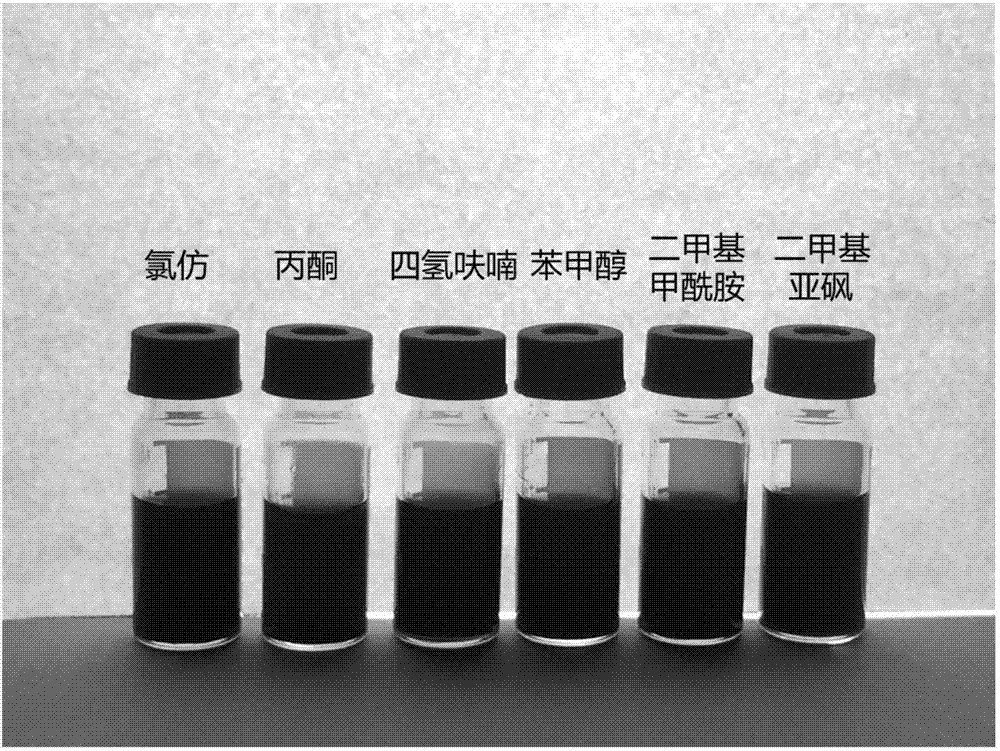
[0062] The hyperbranched polytriazole functionalized graphene prepared in Example 1 has a field scanning electron microscope image as shown in figure 1 As shown, the cloudiness in the figure shows that the hyperbranched polytriazole is well modified on the graphene surface, and the graphene sheets are well peeled off; the hyperbranched polytriazole functionalized graphene is dispersed in different solvents Situation like figure 2 Shown from figure 2 It can be seen that it is well dispersed in common organic solvents such as chloroform, acetone, tetrahydrofuran, benzyl alcohol, dimethylformamide, and dimethyl sulfoxide.
[0063] The preparation method of hyperbranched polytriazole functi...
Embodiment 2
[0073] Hyperbranched polytriazole functionalized graphene, the end of the hyperbranched polytriazole is grafted with aromatic groups, and then uniformly modified to the surface of the graphene through non-covalent bonding. The aromatic group is The non-covalent bond function is π-π conjugation.
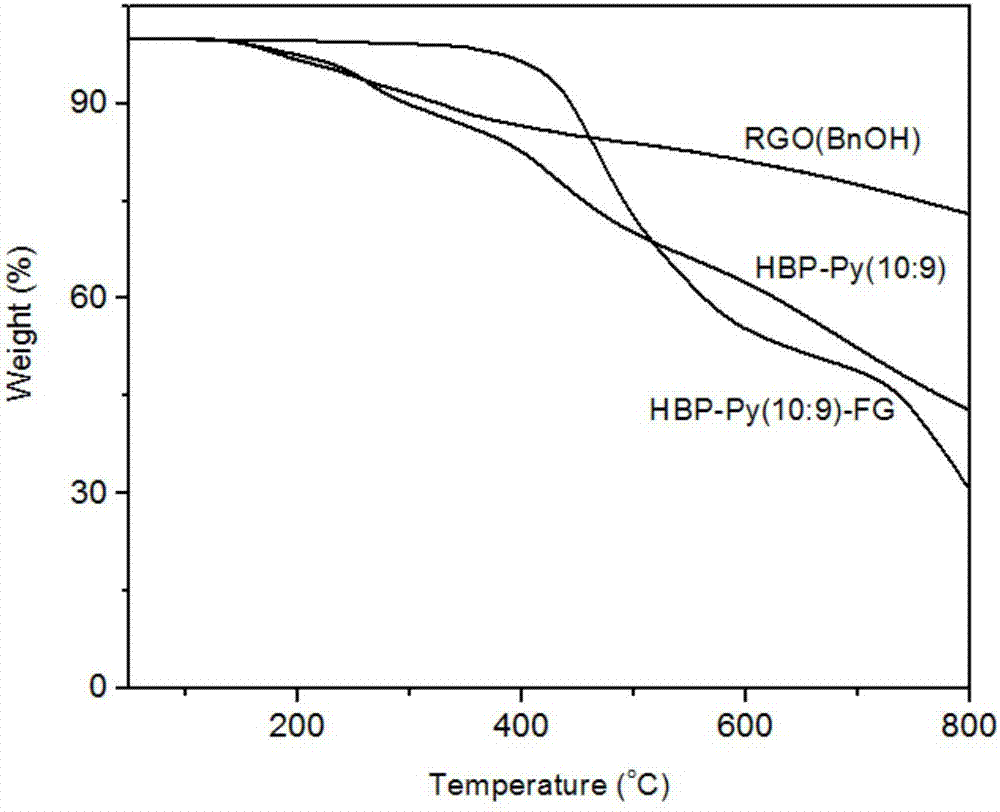
[0074] The thermal weight loss curves of hyperbranched polytriazole functionalized graphene and reduced graphene oxide prepared in Example 2 are as follows image 3 As shown, the grafting amount of the hyperbranched polymer is 45.8%. Such as image 3 As shown, the hyperbranched polytriazole has a good coating on the surface of graphene, which is beneficial to the further modification of graphene.
[0075] The preparation method of hyperbranched polytriazole functionalized graphene in Example 2 is as follows:
[0076] (1) Dissolve 10 g of 3,5-dihydroxybenzoic acid in 150 mL of methanol, add 1.2 mL of hydrochloric acid, and react at 90°C for 24 hours. The methanol is rotary evaporated. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com