A method for improving the antibacterial performance of porcine lysozyme by fusing the hlh functional domain at the N-terminus
A lysozyme, N-terminal technology, applied in the field of bioengineering, can solve the problems of limited application and no bacteriostatic effect, and achieve the effect of broadening the antibacterial spectrum and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the production of fusion porcine lysozyme
[0032] 1. The coding gene of the functional domain HLH (amino acid sequence shown in SEQ ID NO.2) simulated by Swiss-Model software is fused with the N-terminal of the porcine lysozyme coding gene, and no cleavage site is added between the two genes , the amino acid sequence and nucleotide sequence of the fused gene are respectively shown in SEQ ID NO.4 and SEQ ID NO.5.
[0033] 2. Ligate the fused gene sequence with the expression vector pET-28a(+), use restriction enzymes BamH Ⅰ and Hind Ⅲ for double digestion, transfer it into the host E.coli BL21(DE3) for expression, and store at 30°C, Under the condition of 200r / min, 0.1mmol / L IPTG was used to induce for 8h, and the fermentation broth was centrifuged to obtain bacterial cells. It was ultrasonically crushed to obtain recombinant protein inclusion bodies.
[0034] 3. The inclusion bodies obtained above were refolded by the dilution method, and the solution u...
Embodiment 2
[0036] Example 2: Detection of antibacterial properties of fusion lysozyme
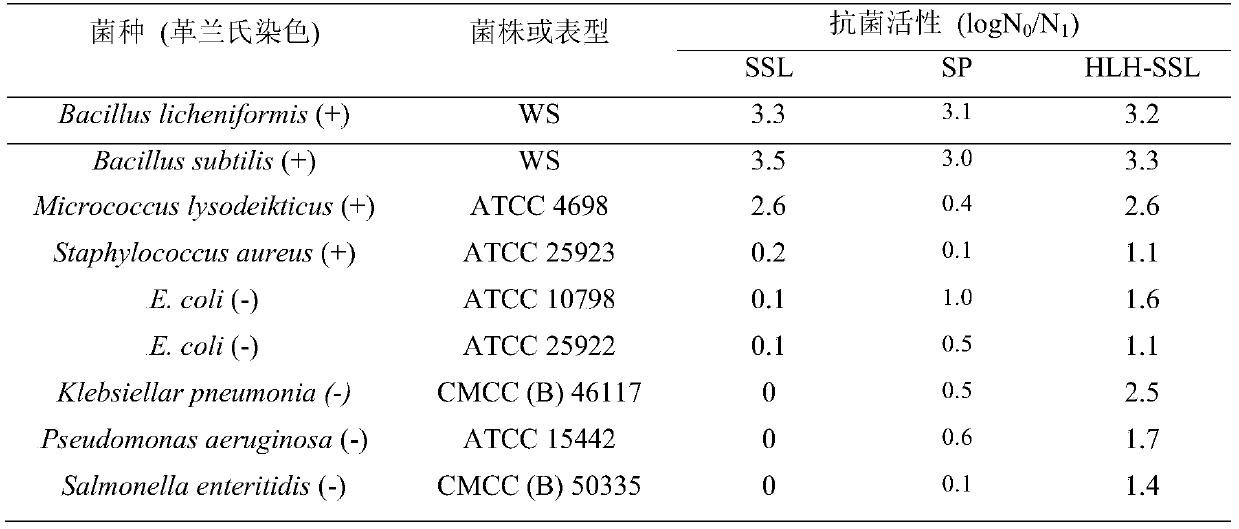
[0037] The antibacterial activity of the fusion porcine lysozyme was determined according to the method in the literature. After secondary activation, the test bacteria were inoculated into a Erlenmeyer flask containing 30mL TSB medium with 1% inoculum and cultivated to OD 600 0.6, take 0.2mL bacterial liquid and mix with 0.4mL TSB medium, add 0.2mL PBS (0.05mol / L, pH 7.0) buffer solution containing fusion lysozyme (porcine lysozyme SSL and antimicrobial peptide SP as control) and mix well , so that the final concentration of fusion lysozyme (or control) is 8.3 × 10 -8mol / L. After the mixed system was cultured at 37°C and 200r / min for 2 hours, it was diluted and spread on a TSB plate, and counted after the colonies grew. Calculation of the inhibition coefficient log N 0 / N 1 , where N 0 Refers to the number of colonies in the blank group, that is, only PBS solution is added; N 1 is the number o...
Embodiment 3
[0041] Embodiment 3: the application of fusion lysozyme
[0042] The fusion lysozyme of the invention can not only resist various Gram-positive bacteria, but also kill various Gram-negative bacteria, including various pathogenic bacteria, and can be used as a feed additive instead of antibiotics.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com