Carbon nitride nanotube and preparation method thereof
A technology of nanotubes and carbon nitride, which is applied in the field of nanomaterials, can solve the problems of materials harmful to the environment and health, not suitable for large-scale production, and the size of nanotubes is not good, and achieves low cost, high yield, and a preparation method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
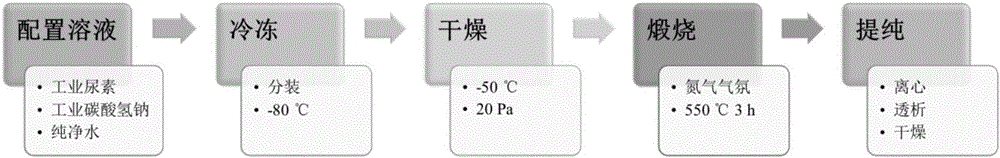
[0032] (1) Preparation of solution: Dissolve urea and sodium bicarbonate in 100 mL of deionized water at a molar ratio of 40:1, fully stir and mix evenly to prepare a colorless and transparent solution with a urea concentration of 6 mol / L;
[0033](2) Freezing: freeze the colorless transparent solution obtained in step (1) in a low-temperature environment, the freezing temperature is -80°C, and the freezing time is 30 hours, so that the solution is frozen as a whole to obtain a uniform white blocky solid;
[0034] (3) Drying: The white massive solid obtained in step (2) is quickly transferred to a vacuum freeze dryer, and freeze-dried at a vacuum degree of ≤20Pa and a freezing temperature of -80°C. The freeze-drying time is 48h, making the white Block solid freeze-dried to constant weight;
[0035] (4) Calcination: Place the white blocky solid obtained in step (3) in a crucible with a cover, and place it in a nitrogen atmosphere furnace, heat up to a calcination temperature of...
Embodiment 2
[0039] (1) Preparation of solution: urea and sodium bicarbonate were dissolved in 50 mL of deionized water at a molar ratio of 5:1, fully stirred and mixed evenly to prepare a colorless and transparent solution with a urea concentration of 3 mol / L;
[0040] (2) Freezing: freeze the colorless transparent solution obtained in step (1) in a low-temperature environment, the freezing temperature is -80°C, and the freezing time is 30 hours, so that the solution is frozen as a whole to obtain a uniform white blocky solid;
[0041] (3) Drying: The white massive solid obtained in step (2) is quickly transferred to a vacuum freeze dryer, and freeze-dried at a vacuum degree of ≤20Pa and a freezing temperature of -70°C. The freeze-drying time is 48h, and the white Block solid freeze-dried to constant weight;
[0042] (4) Calcination: Place the white blocky solid obtained in step (3) in a crucible with a lid, and place it in a nitrogen atmosphere furnace, heat up to a calcination temperatu...
Embodiment 3
[0045] (1) Preparation of solution: urea and sodium bicarbonate were dissolved in 100mL deionized water at a molar ratio of 10:1, fully stirred and mixed evenly to prepare a colorless and transparent solution with a urea concentration of 4mol / L;
[0046] (2) Freezing: freeze the colorless transparent solution obtained in step (1) in a low-temperature environment, the freezing temperature is -80°C, and the freezing time is 24 hours, so that the solution is frozen as a whole to obtain a uniform white blocky solid;
[0047] (3) Drying: The white massive solid obtained in step (2) is quickly transferred to a vacuum freeze dryer, the vacuum degree is set to 8Pa, freeze-dried at a freezing temperature of -80°C, and the freeze-drying time is 48h. The white lumpy solid was freeze-dried to constant weight;
[0048] (4) Calcination: Place the white blocky solid obtained in step (3) in a crucible with a lid, and place it in a nitrogen atmosphere furnace, heat up to a calcination temperat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com