A kind of synthetic method of 2'-deoxyadenosine
A technology of deoxyadenosine and its synthetic method, which is applied in the fields of drug synthesis and intermediate preparation, can solve problems such as inability to apply industrial production, unavoidable column chromatography, difficult industrial production, etc., and achieve simplified operation, low cost and stable product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
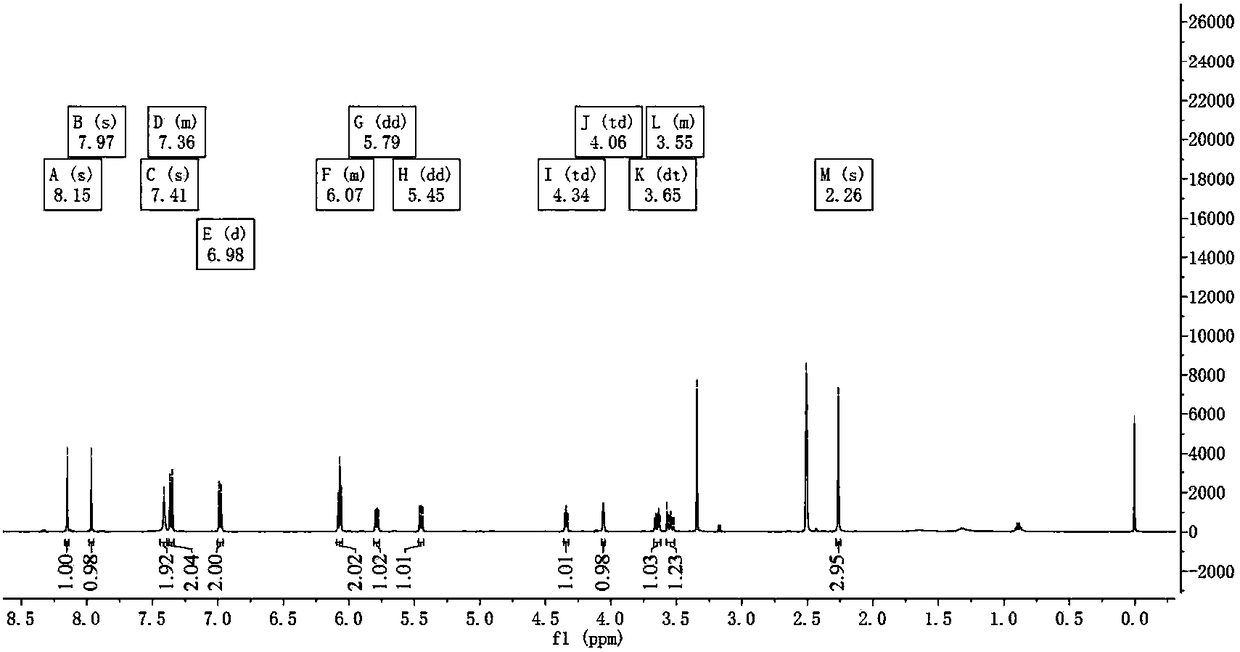
[0073] (1) Synthesis of 2'-O-tosyladenosine (III) from adenosine (I)
[0074] Weigh 1.34g of adenosine, 1.37g of di-n-butyltin oxide, and 35ml of methanol, put the above-mentioned raw materials in a 100ml round bottom flask, heat to reflux for 2h, and the resulting 2',3'-di-n-butylstannylidene Glycoside (II) is directly subjected to the next step reaction, adding 3.10 g of p-toluenesulfonyl chloride and 2 ml of triethylamine to the reaction mixture, stirring and reacting at room temperature for 3-5 h, standing at room temperature for 1 h after the reaction, suction filtering, and washing the filter residue with methanol, and then The filter residue was dried in a vacuum drying oven to finally obtain 1.71 g of white powdery solid 2'-O-p-toluenesulfonyladenosine with a yield of 81.2%. HPLC purity 98.1%.
[0075] (2) Synthesis of 2'-deoxyadenosine (IV) from 2'-O-tosyladenosine (III)
[0076] Weigh 1.69g of 2'-O-p-toluenesulfonyladenosine, 10ml of anhydrous dimethyl sulfoxide, p...
Embodiment 2
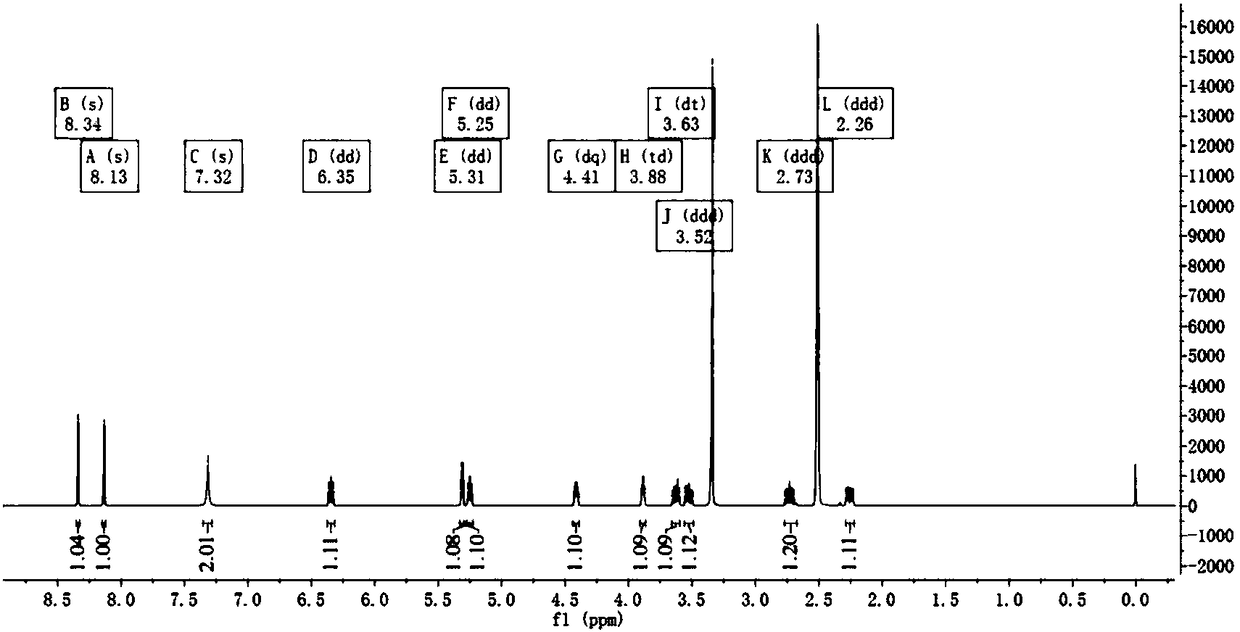
[0078] (1) Synthesis of 2'-O-tosyladenosine (III) from adenosine (I)
[0079] Weigh 2.67g of adenosine, 2.99g of di-n-butyltin oxide, and 50ml of methanol, put the above-mentioned raw materials in a 100ml round bottom flask, heat to reflux for 3h, remove the methanol by rotary evaporation after reaction, and generate 2',3'-di n-Butylstannylidene adenosine (II) directly proceeds to the next step reaction, add 3.10g of p-toluenesulfonyl chloride, 2ml of triethylamine, and 50ml of dichloromethane to the reaction mixture, stir and react at room temperature for 5-7h, and rotate to evaporate after reaction Concentrate, add 15ml of methanol, stand at room temperature for 1h, filter with suction, wash the filter residue with methanol, and then dry the filter residue in a vacuum drying oven to finally obtain 3.76g of white powdery solid 2'-O-p-toluenesulfonyladenosine. The rate is 89.2%. HPLC purity 98.6%.
[0080] (2) Synthesis of 2'-deoxyadenosine (IV) from 2'-O-tosyladenosine (III...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com