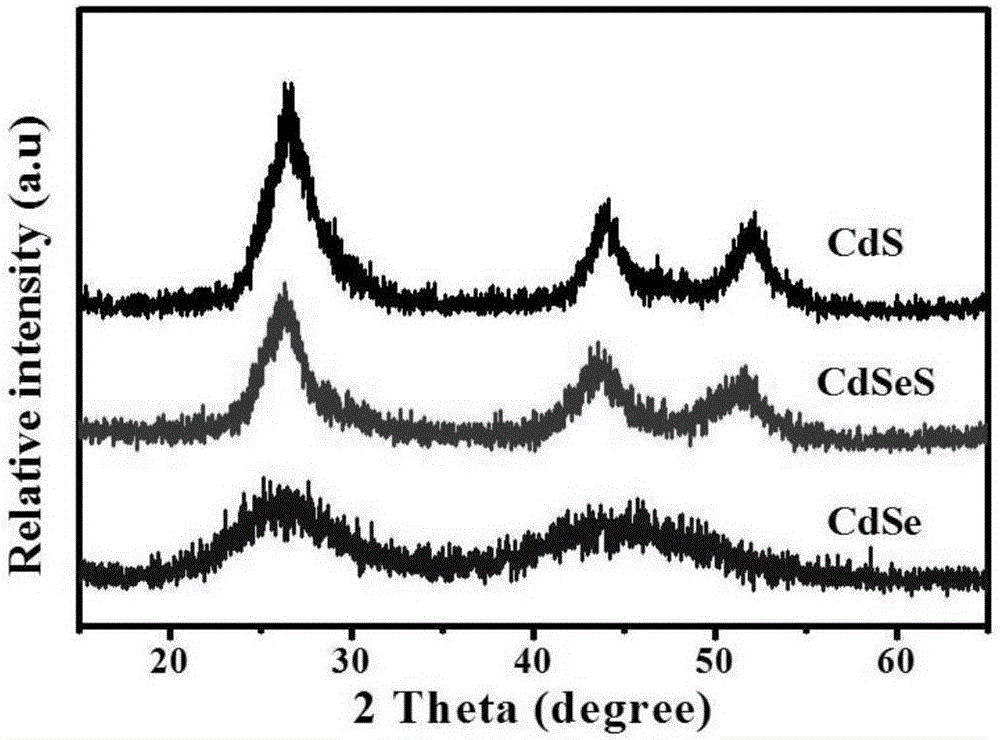
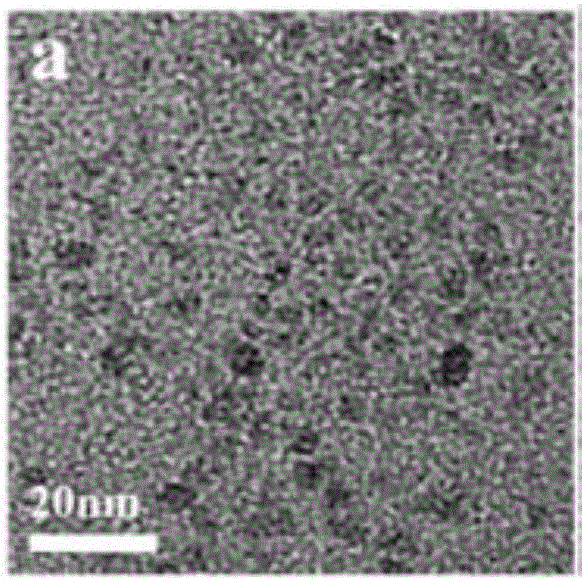
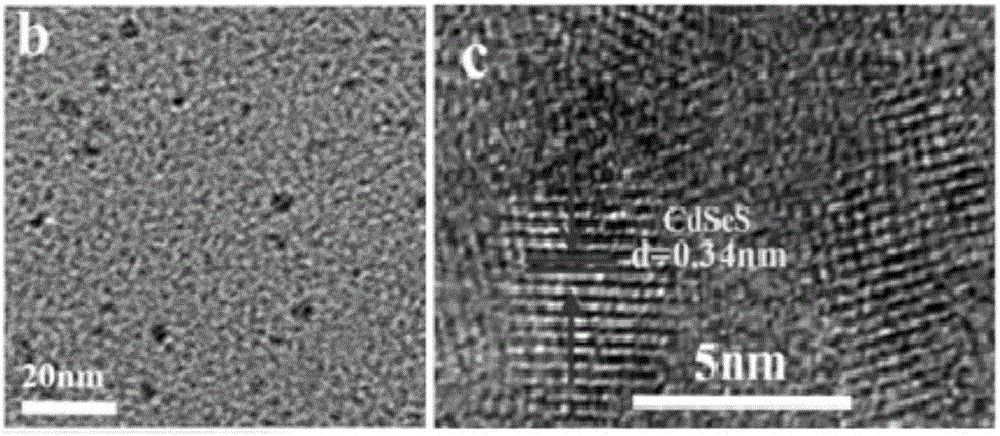
Cadmium sulphide selenide solid solution quantum dot and preparation method and photocatalytic hydrogen production application thereof
A technology of quantum dots and solid solution, applied in the field of photocatalytic materials, can solve the problems of complex process and difficult to obtain products, and achieve the effects of simple process, improved solar energy utilization rate, and huge specific surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of CdSeS solid solution quantum dots:
[0038] (1) CdSe precursor Na 2 SeSO 3 Preparation: Sodium sulfite and selenium powder were added to deionized water at a mass ratio of 3:1 and refluxed at 90°C for 10 hours to obtain Na with a concentration of 0.2mol / L. 2 SeSO 3 The solution is ready for use;
[0039] (2) Preparation of water-soluble CdSe quantum dots: 0.272g (CH 3 COO) 2 Cd·2H 2 O was dissolved in 180mL distilled water and stirred until the resulting solution had a concentration of 5mg / L, then 0.4mol / L NaOH solution was added dropwise until the pH of the solution was 11, and then reacted with high-purity nitrogen for 30 minutes, and then 240μL thioglycolic acid and 3mL were added respectively Na at a concentration of 0.2mol / L 2 SeSO 3Solution, reacted at room temperature for 1 hour, then heated to 60°C and stirred for 1 hour to obtain a water-soluble CdSe quantum dot suspension (concentration: 780mg / L), and added 30mL of acetone to the 20mL wa...
Embodiment 2
[0053] Preparation of CdSeS quantum dots:
[0054] (1) CdSe precursor Na 2 SeSO 3 Preparation: Sodium sulfite and selenium powder were added to deionized water at a mass ratio of 3:1 and refluxed at 70°C for 5 hours to obtain Na with a concentration of 0.1mol / L. 2 SeSO 3 solution;
[0055] (2) Preparation of water-soluble CdSe quantum dots: 0.09g (CH 3 COO) 2 Cd·2H 2 O was dissolved in 180 mL of distilled water and stirred to obtain a solution concentration of 0.5 mg / L. Then add 0.4mol / L NaOH solution dropwise until the pH of the solution is 11, then pass high-purity nitrogen to react for 30 minutes, then add 240μL thioglycolic acid and 3mL NaOH with a concentration of 0.1mol / L 2 SeSO 3 Solution, reacted at room temperature for 1 hour, then heated to 40°C and stirred for 40 minutes to obtain a water-soluble CdSe quantum dot suspension (concentration is 500mg / L), and added 30mL of acetone to the 20mL water-soluble CdSe quantum dot suspension that was taken out, and allo...
Embodiment 3
[0058] Preparation of CdSeS quantum dots: in a beaker containing 80mL of distilled water, add 10mL of the water-soluble CdSe quantum dot suspension prepared in Example 1 and 5.24g Na 2 S with 2.5g NaSO 3 , the reactants in the beaker were ultrasonically decomposed, mixed evenly and stirred at room temperature for 40 minutes, and dried by centrifugation to obtain CdSeS solid solution quantum dots.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com