Branched side chain polymer anion exchange membrane and preparation method thereof
An anion exchange membrane and polymer technology, applied in the field of branched side chain polymer anion exchange membrane and its preparation, can solve the problems of decreased solubility, low controllability of alkyl side chain length, entanglement of main chain and side chain and other issues, to achieve high chemical reactivity, taking into account the effect of conductivity-stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
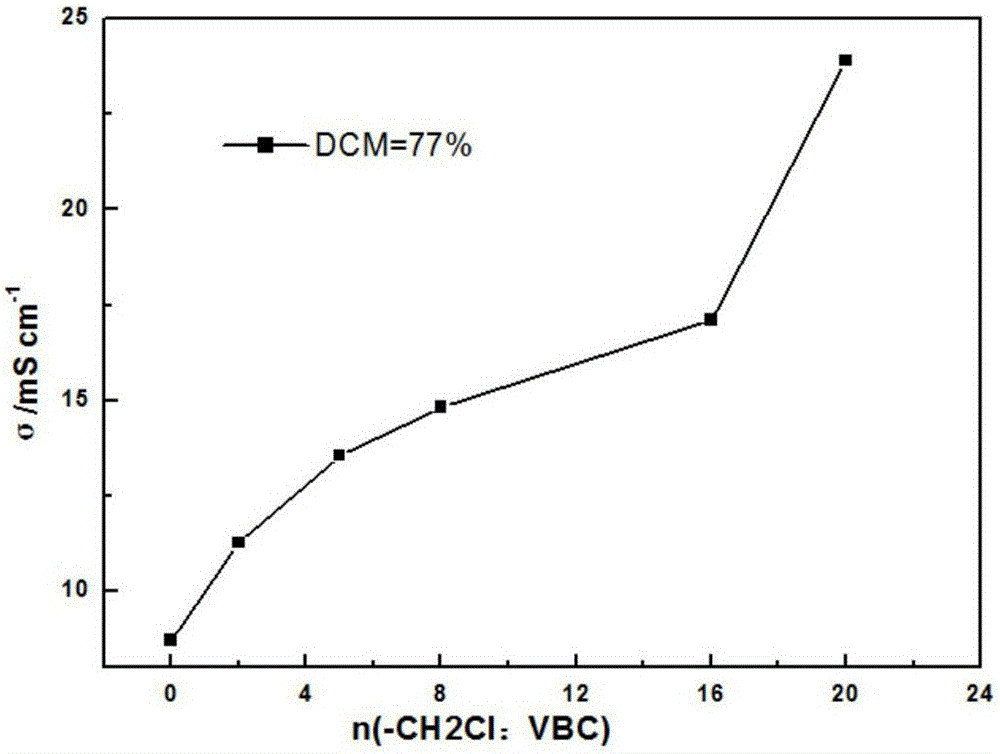
[0030]Synthesis of chloromethylated polysulfone (CMPSf): under nitrogen protection, 7 g of polysulfone was dissolved in chloroform, and 420 μl of SnCl was added dropwise 4 , and then slowly add 11.9 ml of chloromethyl methyl ether with a syringe; react at 55°C for 10 hours, after the reaction, precipitate the mixture in ethanol, filter it with suction, soak it in ethanol and stir for 12 hours, filter it with suction, and use it several times After washing with deionized water and ethanol, the resulting white precipitate was vacuum-dried at 60°C for 24 hours. The degree of chloromethylation (DCM) of the obtained product was 0.77.
[0031] Ionization of chloromethylated polysulfone (CMPSf): Take 1 mole part of CMPSf dissolved in 25 mole parts of DMAC, add 1 mole part of 1-methylimidazole (MIm), and react at 60°C for 6h.
[0032] Membrane casting: cast the above reaction solution on a clean glass plate, flow to form a membrane, and dry at 60°C for 18 hours to obtain an anion mem...
Embodiment example 2
[0035] Synthesis of chloromethylated polysulfone (CMPSf): under nitrogen protection, 7 g of polysulfone was dissolved in chloroform, and 420 μl of SnCl was added dropwise 4 , and then slowly add 11.9 ml of chloromethyl methyl ether with a syringe; react at 55°C for 10 hours, after the reaction, precipitate the mixture in ethanol, filter it with suction, soak it in ethanol and stir for 12 hours, filter it with suction, and use it several times After washing with deionized water and ethanol, the resulting white precipitate was vacuum-dried at 60°C for 24 hours. The degree of chloromethylation (DCM) of the obtained product was 0.77.
[0036] Synthesis of branched side chain polymer (BPSf) by ATRP reaction: Weigh about 0.6g of CMPSf with DCM=77%, and NMP (10ml) as solvent, dissolve in a four-necked bottle, in order to ensure nitrogen protection environment, nitrogen gas-pump After vacuum-nitrogen circulation for 3 to 5 times, quickly add 0.02g of cuprous chloride (CuCl) and 0.09g...
Embodiment example 3
[0041] Synthesis of chloromethylated polysulfone (CMPSf): under nitrogen protection, 7 g of polysulfone was dissolved in chloroform, and 420 μl of SnCl was added dropwise 4 , and then slowly add 11.9 ml of chloromethyl methyl ether with a syringe; react at 55°C for 10 hours, after the reaction, precipitate the mixture in ethanol, filter it with suction, soak it in ethanol and stir for 12 hours, filter it with suction, and use it several times After washing with deionized water and ethanol, the resulting white precipitate was vacuum-dried at 60°C for 24 hours. The degree of chloromethylation (DCM) of the obtained product was 0.77.
[0042] Synthesis of branched side chain polymer (BPSf) by ATRP reaction: Weigh about 0.6g of CMPSf with DCM=77%, NMP (13ml) as solvent, dissolve in a four-necked bottle, in order to ensure nitrogen protection of the environment, nitrogen-pumping After vacuum-nitrogen circulation for 3 to 5 times, quickly add 0.05g of cuprous chloride (CuCl) and 0.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com