Pseudomonas aeruginosa ZJPH1504 and application thereof in preparation of sitagliptin chiral intermediate
A technology of Pseudomonas aeruginosa and sitagliptin, applied in the direction of bacteria, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of poor solubility of substrates and low yields, and achieve shortened reaction time, Improved reaction yield and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: For catalytic reduction of 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine Screening of microbial strains of -7-(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one
[0034] Enrichment culture: Add 1g of fresh soil samples (collected from the campus of Zhejiang University of Technology (Hangzhou, Zhejiang)) into a 250mL shaker flask filled with 50mL of enrichment medium, culture at 30°C and 200rpm for 5 days, and let stand. Take 1 mL of the culture medium and transfer it to a fresh enrichment medium, continue to cultivate for 5 days, and repeat the enrichment process 3 times. The enrichment medium consists of: (NH 4 ) 2 SO 4 2g / L, KH 2 PO 4 1g / L, NaCl 0.5g / L, MgSO 4 ·7H 2 O 0.5g / L, the compound of formula (II) (2g / L) is the sole carbon source, the solvent is water, pH 6.5, and sterilized at 120°C for 20min.
[0035] Plate primary screening: the enriched culture solution was diluted 10 with normal saline 4 -10 6 After doubling, it w...
Embodiment 2
[0050] Example 2: Determination of the chiral configuration of the reduced product of strain ZJPH1504
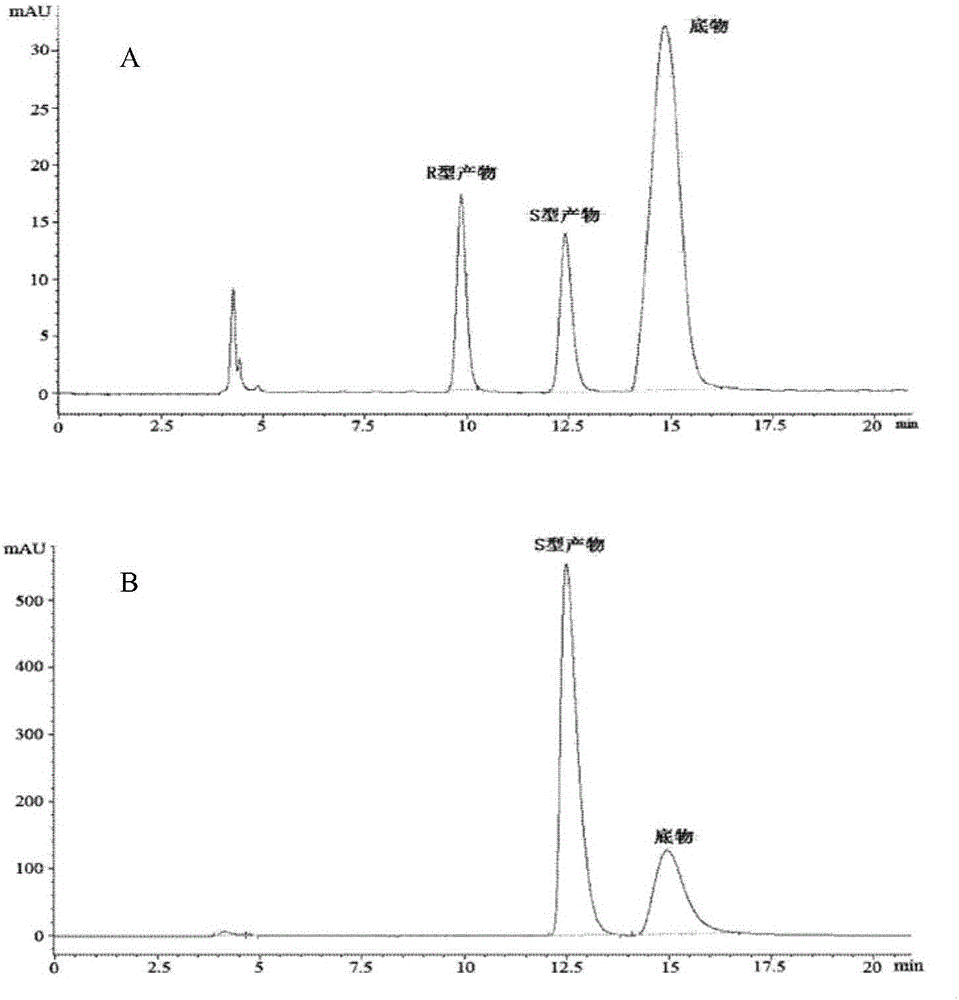
[0051] With reference to the separation method of related substances described in the patent 201510289729.X (publication number CN 104893989 A), the biotransformation solution of the bacterial strain ZJPH1504 obtained in Example 1 was extracted twice with an equal volume of ethyl acetate, the solvent was removed by rotary evaporation, and then passed Preparative thin-layer chromatography (developing agent is ethyl acetate: sherwood oil: glacial acetic acid=80:20:0.1 (v / v / v); TLC plate is GF254; Color develops under the ultraviolet of wavelength 254nm) to separate, collect R f A product with a value of 0.44 gave the reduced product compound of formula (I). This substance corresponds to one of the peaks in the HPLC chromatogram of the compound of racemic formula (I), see image 3 . According to the standard method, the specific optical rotation (SOR) of the reduced product ...
Embodiment 3
[0052] Example 3: Obtaining of Resting Cell Enzyme Sources under Shake Flask Culture Conditions
[0053] (1) Slant culture: Pseudomonas aeruginosa ZJPH1504 was inoculated into the slant medium, and cultured at 30° C. for 36 hours to obtain mature cultured slant strains. The final concentration of the slant medium consists of: glucose 15g / L, peptone 7.5g / L, yeast extract 6g / L, (NH 4 ) 2 SO 4 3g / L, KH 2 PO 4 1.5g / L, NaCl 0.75g / L, MgSO 4 ·7H 2 O 0.75g / L, agar powder 20g / L, solvent is water, pH 6.5.
[0054] (2) Seed culture: inoculate the slant bacteria into the seed culture medium, cultivate at 30° C. and 200 rpm for 12 hours, and obtain the seed liquid. The final concentration of the seed medium consists of: glucose 15g / L, peptone 7.5g / L, yeast extract 6g / L, (NH 4 ) 2 SO 4 3g / L, KH 2 PO 4 1.5g / L, NaCl 0.75g / L, MgSO 4 ·7H 2 O 0.75g / L, solvent is water, pH 6.5.
[0055] (3) Fermentation culture: inoculate the seed liquid into the fermentation medium with an ino...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com