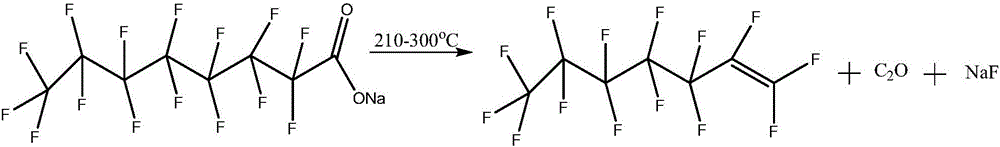
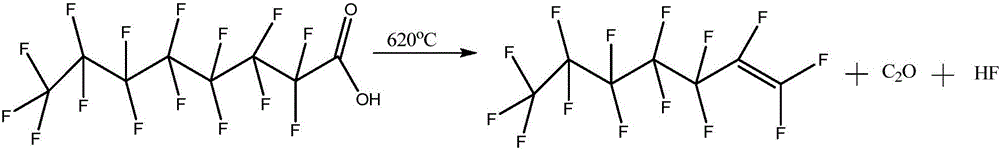
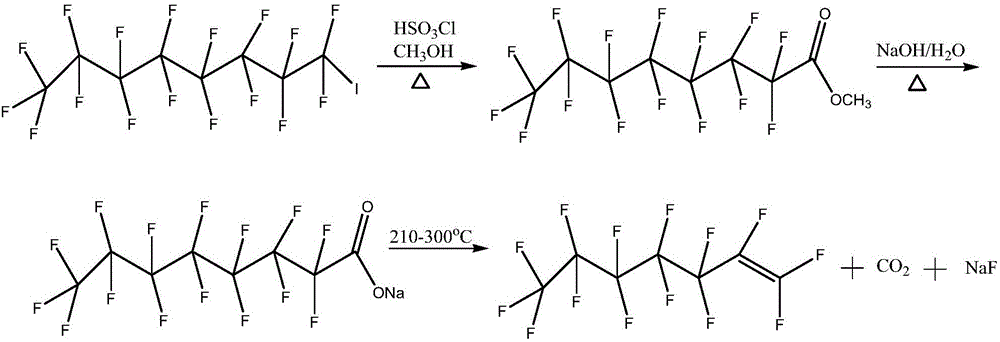
Method for preparing perfluoroheptene isomers
A technology for isomers and perfluoroheptene, applied in the field of preparing perfluoroheptene isomers, can solve the problems of expensive raw materials, high energy consumption, difficult to obtain, etc., and achieves mild reaction conditions, easy industrialization, The effect of easy response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] At normal pressure, under stirring conditions, in a 250 ml glass flask equipped with a condenser tube and a bubbler (making the reaction at normal pressure), add catalyst cesium fluoride, solvent dimethylformamide and raw material perfluoro-1-heptane ene, the molar ratio of cesium fluoride / dimethylformamide / perfluoro-1-heptene is 0.3 / 12 / 1, the reaction temperature is 50°C, and the reaction time is 3 hours. After the reaction, wash 100ml × 3 times to remove the solvent, dry with 2.0g of anhydrous magnesium sulfate, filter to obtain the organic phase, take the organic phase and carry out gas chromatography to analyze the composition of the organic matter. The results are shown in Table 1.
[0044] The above organic phase is rectified to obtain perfluoro-1-heptene, E-perfluoro-2-heptene, Z-perfluoro-2-heptene, E-perfluoroheptene isomers of perfluoroheptene Fluoro-3-heptene and Z-perfluoro-3-heptene. Among them, the boiling point of E-perfluoro-3-heptene is 69-71°C (760mmH...
Embodiment 2
[0046] The same operation as in Example 1, except that the molar ratio of cesium fluoride / dimethylformamide / perfluoro-1-heptene is 0.05 / 20 / 1, and the results are shown in Table 1.
Embodiment 3
[0048] The same operation as in Example 1, except that the molar ratio of cesium fluoride / dimethylformamide / perfluoro-1-heptene is 0.1 / 15 / 1, and the results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com