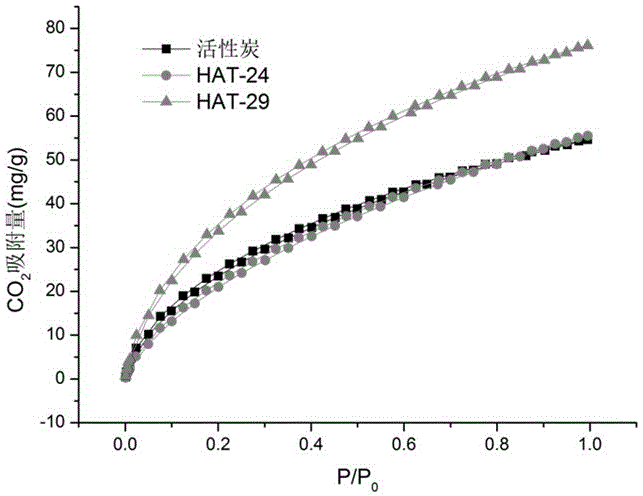
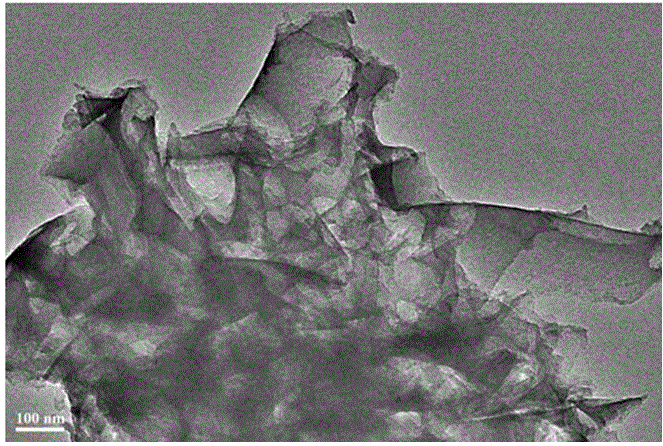
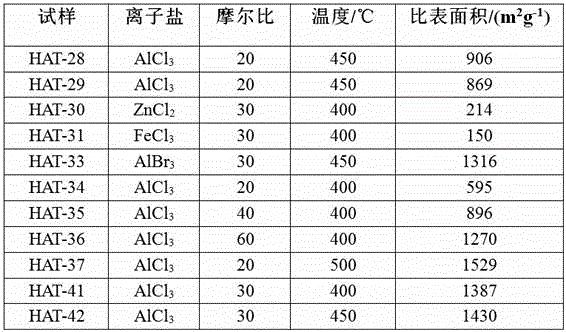
Fused aza-heterocyclic aromatic hydrocarbon porous framework of two-dimensional lamellar structure, and preparation method and application thereof
A technology of heterocondensed aromatic hydrocarbons and porous skeletons, applied in the field of porous immobilized catalysts and their preparation, can solve problems such as limiting prospects, and achieve high reaction efficiency, no by-products, and high reusability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Brunner-Emmet-Teller (BET) specific surface area at 600m 2 / g About the preparation of aza-PAF:
[0028] 1. Preparation of monomer HAT;
[0029] 3mmol o-phenylenediamine and 1mmol cyclohexanone were dissolved in 50mL methanol, and the reaction was refluxed at 80°C for 1h under the action of magnetic stirring. After that, it was lowered to and maintained at room temperature and stirring continued overnight. After the reaction is over, filter to obtain a yellow solid and dry, which is the monomer HAT, ready for use;
[0030] 2. Preparation of aza-PAF catalyst
[0031] Take about 0.05mmol HAT and 1mmol anhydrous AlCl 3 , Added to the thick-walled glass tube. Use an oil pump to pump out the air in the tube, and at the same time seal the glass tube under the torch to make the inside a vacuum condition. The system was placed in a muffle furnace at 400°C for 15 hours. After the reaction, the temperature was lowered to room temperature to obtain a black solid, which was ...
Embodiment 2
[0032] Example 2: BET specific surface area is 900 m 2 Preparation of aza-PAF around / g:
[0033] 1. The preparation of monomer HAT is the same as that described in Example 1-1;
[0034] 2. Preparation of catalyst aza-PAF;
[0035] The preparation process is similar to Example 1-2, except that 0.05mmol HAT is added with 2mmol anhydrous AlCl 3 .
Embodiment 3
[0036] Example 3: BET specific surface area at 1300 m 2 Preparation of aza-PAF around / g:
[0037] 1. The preparation of monomer HAT is the same as that described in Example 1-1;
[0038] 2. Preparation of catalyst aza-PAF;
[0039] The preparation process is similar to Example 1-2, except that 0.05mmolHAT is added with 3mmol anhydrous AlCl 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com