Feeding antibacterial dodecapeptide from pig lysozyme and preparation method of feeding antibacterial dodecapeptide
An antibacterial peptide and lysozyme technology, applied in the field of bioengineering, can solve the problems of limited application and no bacteriostatic effect, and achieve the effects of simple preparation process and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
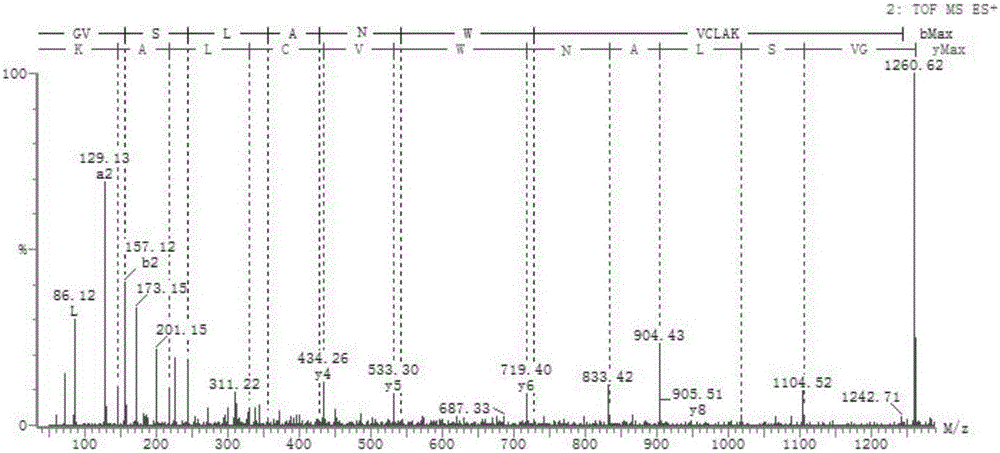
[0028] Embodiment 1: the preparation of antibacterial dodecapeptide
[0029] 1. The lysozyme product is fermented by recombinant Escherichia coli BL21 (DE3), and the coding gene (NCBI-ID: 1174173) is digested by BamHI and HindIII and connected with the vector pET-28a(+), transformed into Escherichia coli BL21 (DE3) induced expression. Under the conditions of 25° C. and 200 r / min, 0.1 mmol / L IPTG was used to induce for 8 hours, and the fermentation broth was centrifuged to obtain bacterial cells. Ultrasonic crushing was performed to obtain the recombinant protein inclusion body, and then the inclusion body was renatured, and finally the biologically active porcine lysozyme was obtained after freeze-drying. The fermented product is hydrolyzed by trypsin (15-30mg / g, trypsin / fermented product)) at 35-38°C for 12-16 hours, after the enzymolysis is completed, boil for 10 minutes to kill the enzyme, and the enzymolyzed solution is ultra-filtered (molecular weight cut-off 3000Da) Co...
Embodiment 2
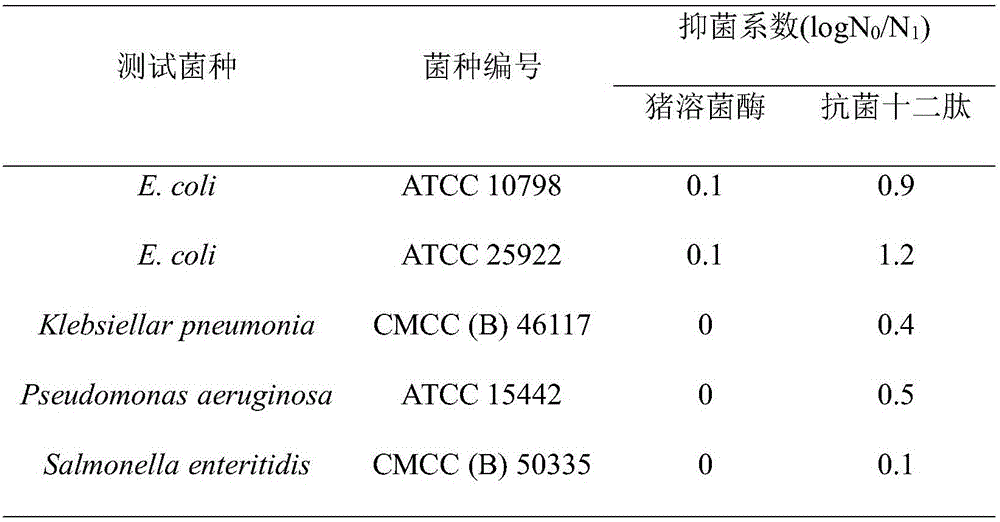
[0037] Embodiment 2: the antibacterial performance detection of antibacterial dodecapeptide
[0038] The antibacterial activity of antibacterial dodecapeptide was determined according to the method in the literature. After secondary activation, the test bacteria were inoculated into a Erlenmeyer flask containing 30mL TSB medium with 1% inoculum and cultivated to OD 600 0.6, take 0.2mL bacterial liquid and mix with 0.4mL TSB medium, add 0.2mL PBS (0.05mol / L, pH 7.0) buffer solution containing antibacterial dodecapeptide (porcine lysozyme as a control) and mix evenly to make the antimicrobial peptide (or control) at a final concentration of 2.5×10- 7 mol / L. After the mixed system was cultured at 37°C and 200r / min for 2 hours, it was diluted and spread on a TSB plate, and counted after the colonies grew. Calculation of the inhibition coefficient log N 0 / N 1 , where N 0 Refers to the number of colonies in the blank group, that is, only PBS solution is added; N 1 is the num...
Embodiment 3
[0042] Embodiment 3: the application of antibacterial dodecapeptide
[0043] The antimicrobial peptide was fused with the porcine lysozyme coding gene to obtain a product with obvious bactericidal effect on both Gram-negative bacteria and positive bacteria, which better made up for the defect of the narrow antibacterial spectrum of porcine lysozyme. The basis for further application is laid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com