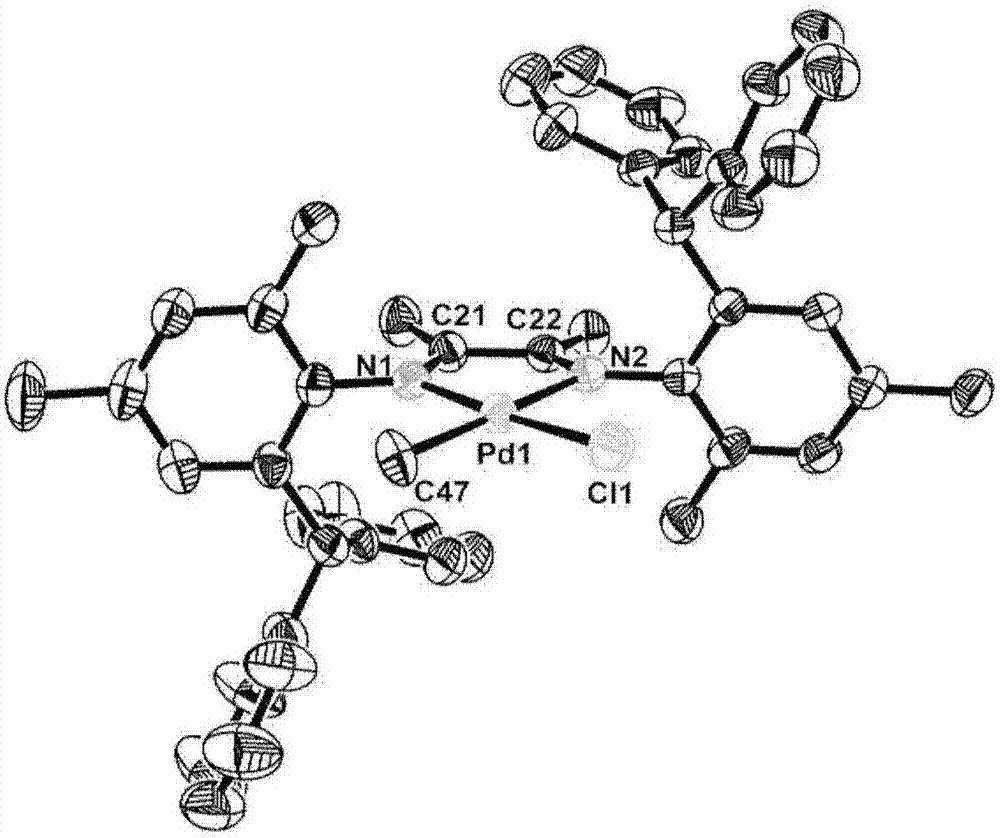
Unsymmetrical diimine palladium catalyst and its ligand, preparation method and use
A technology of catalysts and complexes, applied in the preparation of imino compounds, chemical instruments and methods, palladium organic compounds, etc., can solve the problems of low insertion, low degree of branching, inconvenient processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Synthesis of N,N-bis(2-benzyl-4-methoxy-6-methylphenyl)butane-2,3-diimine
[0073]
[0074] At room temperature, add 8.41 g of 2-benzyl-4-methoxy-6-methylaniline into an eggplant-shaped flask (equipped with a thermometer, reflux condenser and stirrer) containing 200 ml of toluene. 0.86 g of diketone and 20 mg of p-toluenesulfonic acid were heated to 80°C in an oil bath to react for 24 hours. Then, use a water separator (Xinville 14-port water separator) to divide the water and reflux for three days. Use petroleum ether and dichloromethane 1:1 to point the board, and you will find that there is mainly one point, indicating that the reaction has ended. Finally, part of the solvent was removed by rotary evaporation, and then 300 ml of methanol was added to the remaining reaction solution to precipitate a large amount of yellow solid. The solid was obtained by filtration under reduced pressure, washed three times with 20 ml of methanol, and dried under vacuum to ob...
Embodiment 2
[0076] Example 2: Synthesis of N,N-bis(2-benzyl-4-chloro-6-methylphenyl)butane-2,3-diimine
[0077]
[0078] Similar to Example 1, at room temperature, 8.61 g of 2-benzyl-4-chloro-6-methylaniline, 0.86 g of butanedione, and p-methyl were added to an eggplant-shaped flask containing 200 ml of toluene. 20 mg of benzene sulfonic acid, heated to 80°C in an oil bath for 24 hours, and then used a water separator (Xinville 14) to divide the water and reflux for seven days. Use petroleum ether and dichloromethane 1:1 to find the main A point indicates that the reaction has ended. Part of the solvent was removed by rotary evaporation, and then 300 ml of methanol was added to the remaining reaction solution, and a large amount of yellow solid was precipitated. The solid was obtained by filtration under reduced pressure with a water pump, washed three times with 20 ml of methanol, and dried under vacuum to obtain 8.22 g of solid with a yield of 81%.
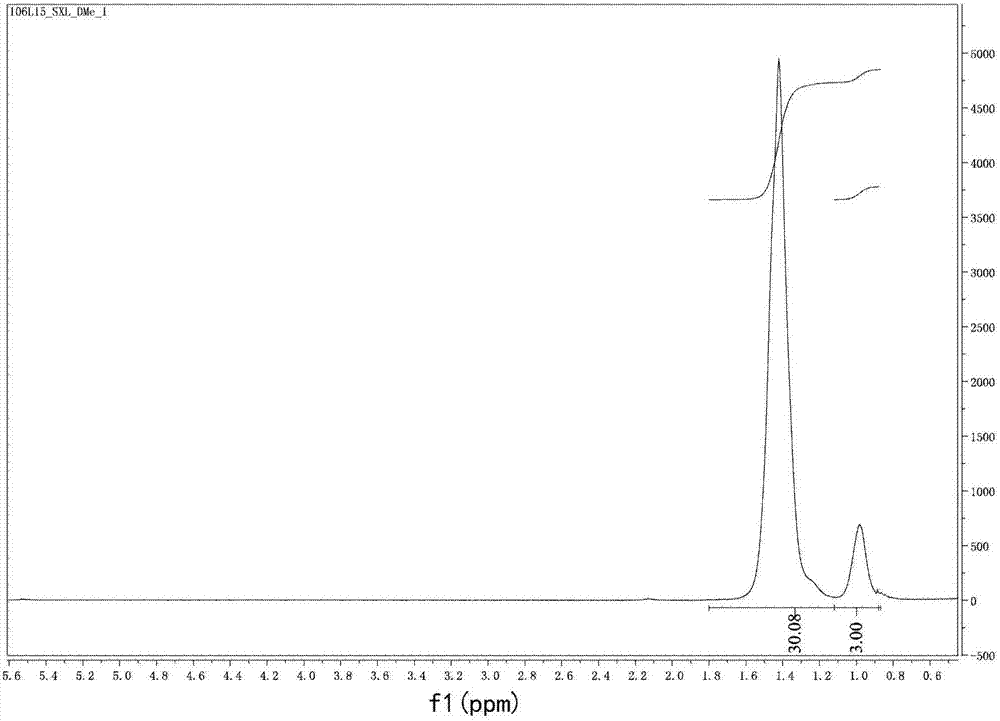
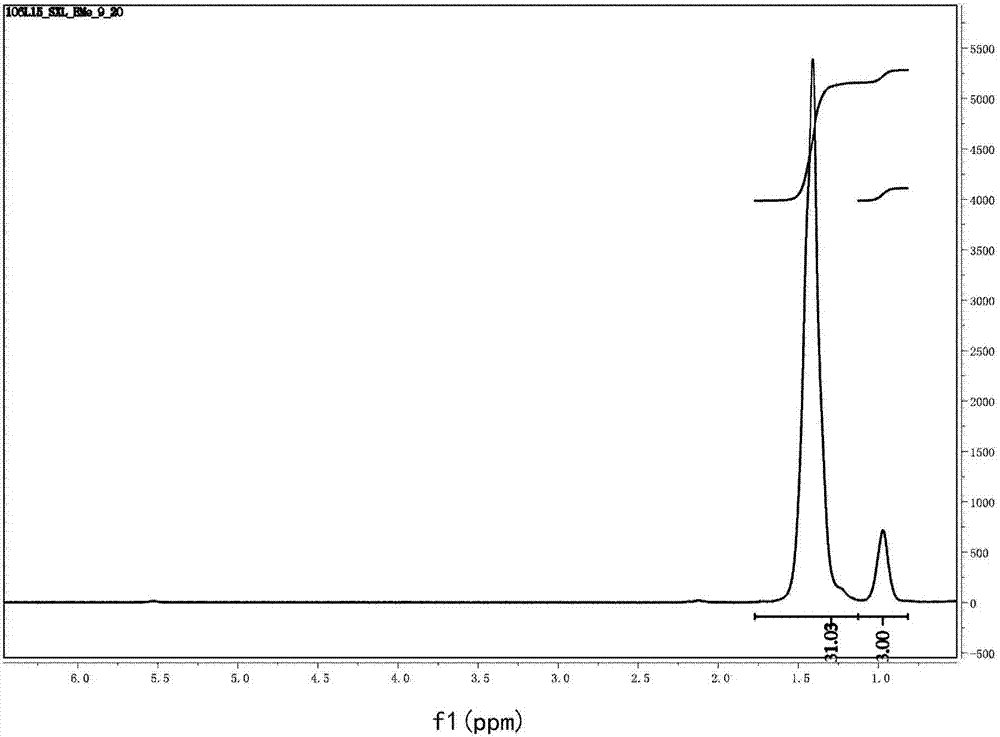
[0079] 1 H NMR(400MHz, CDCl 3 )δ7.27(d,...
Embodiment 3
[0080] Example 3: Synthesis of N,N-bis(2-diphenylmethyl-4-trifluoromethyl-6-methylphenyl)butane-2,3-diimine
[0081]
[0082] Similar to Example 1, at room temperature, add 9.01 g of 2,6-benzyl-4-trifluoromethylaniline, 0.86 g of butanedione and p-methyl to an eggplant-shaped flask containing 200 ml of toluene 20 mg of benzene sulfonic acid, heated to 80°C in an oil bath for 24 hours, and then used a water separator (Xinweier 14) to reflux and divide the water for seven days. Use petroleum ether and methylene chloride 1:1 to point the board and you will find that it is mainly one Click to indicate that the reaction has ended. Part of the solvent was removed by rotary evaporation, and then 300 ml of methanol was added to the remaining reaction solution, and a large amount of yellow solid was precipitated. The solid was obtained by filtration under reduced pressure, washed three times with 20 ml of methanol, and dried under vacuum to obtain 4.03 g of solid with a yield of 50%.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com