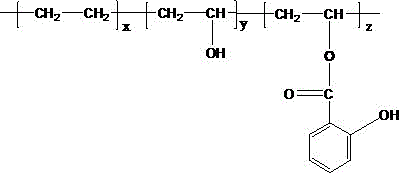Phenolic hydroxyl benzoate based macromolecular antibacterial material preparation method
A technology of phenolic hydroxybenzoic acid and polymer antibacterial agent, which is applied in the field of preparation of polymer antibacterial materials, can solve the problems of difficult migration of antibacterial groups, difficult manufacturing conditions, poor compatibility, etc. Lost, React Device Simple Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] a. In a 250mL four-necked flask, add 39.29g o-hydroxybenzoic acid, 135mL toluene solvent, 30.10mL re-distilled thionyl chloride, 19 drops of N, N-dimethylformamide as catalyst, 55°C After constant temperature reaction for 3 hours, unreacted thionyl chloride and toluene solvent were removed to obtain 44.09 g of o-hydroxybenzoyl chloride, and the conversion (yield) rate of o-hydroxybenzoyl chloride was 98.98%. Its specific structural formula is:
[0027]
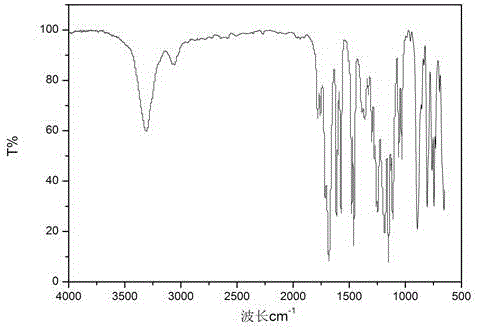
[0028] The infrared spectrum of o-hydroxybenzoyl chloride is as follows figure 1 shown.
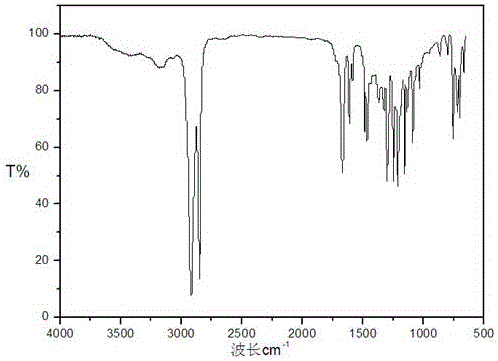
[0029] b. Add 76.11g EVOH (the mass content of vinyl alcohol is 16.60%) into a 1L four-neck flask equipped with a stirring, reflux condenser, and tail gas absorption device, add 385mL tetrahydrofuran, and raise the temperature to completely dissolve it; o-Hydroxybenzoyl chloride was dissolved in 60mL tetrahydrofuran solvent and added dropwise to the above solution, and refluxed for 8 hours; the reaction solution was poured int...
Embodiment 2
[0033] a. In a 250mL four-necked flask, add 39.29g o-hydroxybenzoic acid, 135mL toluene solvent, 30.10mL re-distilled thionyl chloride, 19 drops of N, N-dimethylformamide as catalyst, 55°C After constant temperature reaction for 3 hours, unreacted thionyl chloride and toluene solvent were removed to obtain 44.09 g of o-hydroxybenzoyl chloride, and the conversion rate of o-hydroxybenzoyl chloride was 98.98%. Its concrete structural formula is with embodiment 1.
[0034] b. Add 58.54g EVOH (the mass content of vinyl alcohol is 16.60%) into a 500mL four-neck flask equipped with stirring, reflux condenser, and tail gas absorption device, add 293mL tetrahydrofuran, and raise the temperature to completely dissolve it; o-Hydroxybenzoyl chloride was dissolved in 60mL tetrahydrofuran solvent and added dropwise to the above solution, and refluxed for 8 hours; the reaction solution was poured into a large amount of methanol for precipitation, filtered with suction and washed twice with m...
Embodiment 3
[0036]a. In a 500mL four-necked reaction flask, first add 20.00mL of thionyl chloride into 150mL of dioxane to fully dissolve, and then add 12.50g of o-hydroxybenzoic acid into 100mL of dioxane to dissolve, dropwise Added to the above thionyl chloride solution, 0.5h dropwise completed. 30 ° C constant temperature reaction 24h. Distill under reduced pressure to remove unreacted thionyl chloride and dioxane solvent to obtain 14.14 g of light yellow oil, the conversion rate of o-hydroxybenzoyl chloride is 99.79%, add 200 mL of dioxane, mix well, and seal it for storage. Its concrete structural formula is with embodiment 1.
[0037] b. In a 1L four-necked reaction flask, add 25g EVOH (16.60% vinyl alcohol mass content) into 500mL dioxane, fully dissolve at 85°C, and add the o-hydroxybenzoyl chloride solution obtained in step a dropwise Added to the above solution, the system gradually became turbid, reacted at a constant temperature of 85°C for 24h, the reaction solution was pou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com