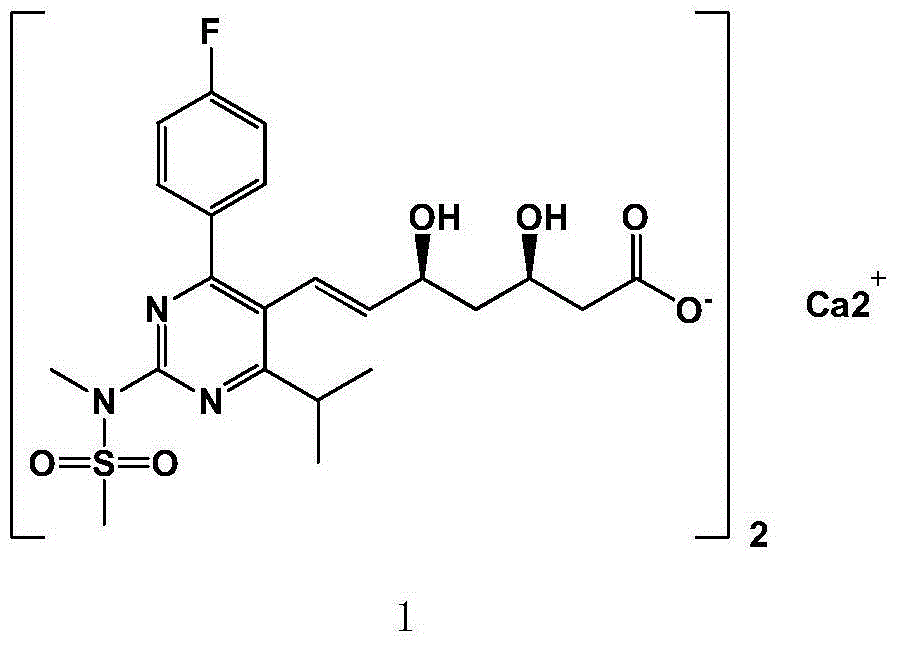
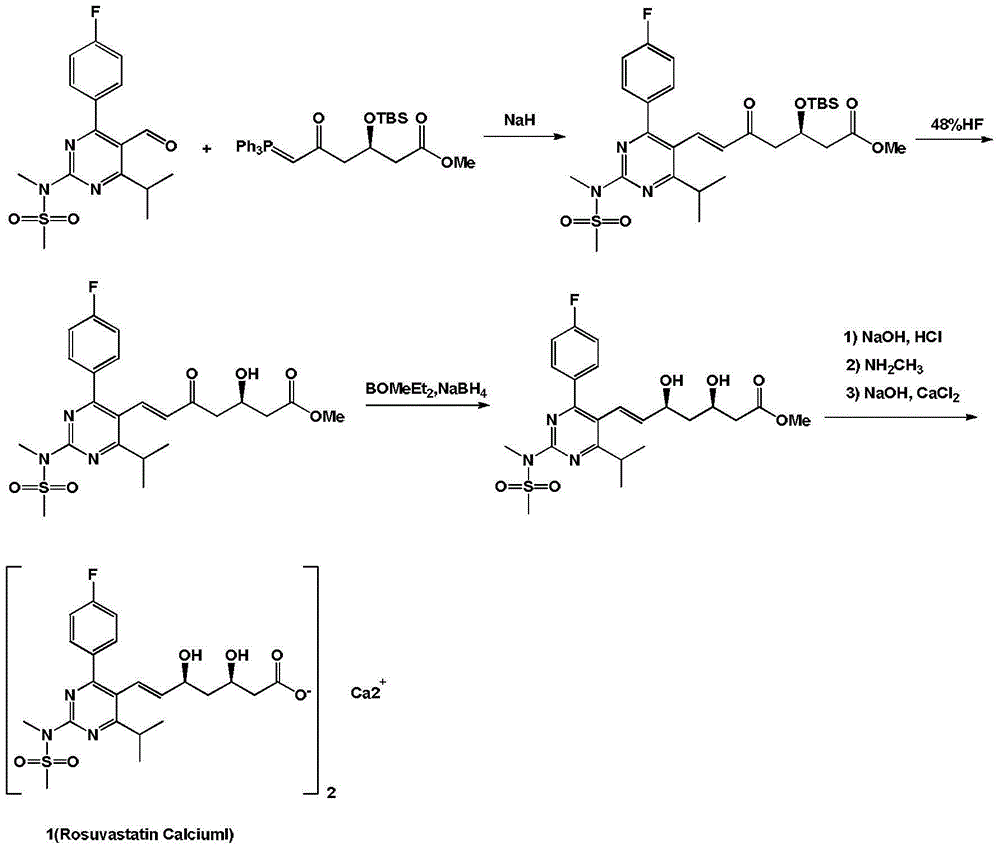
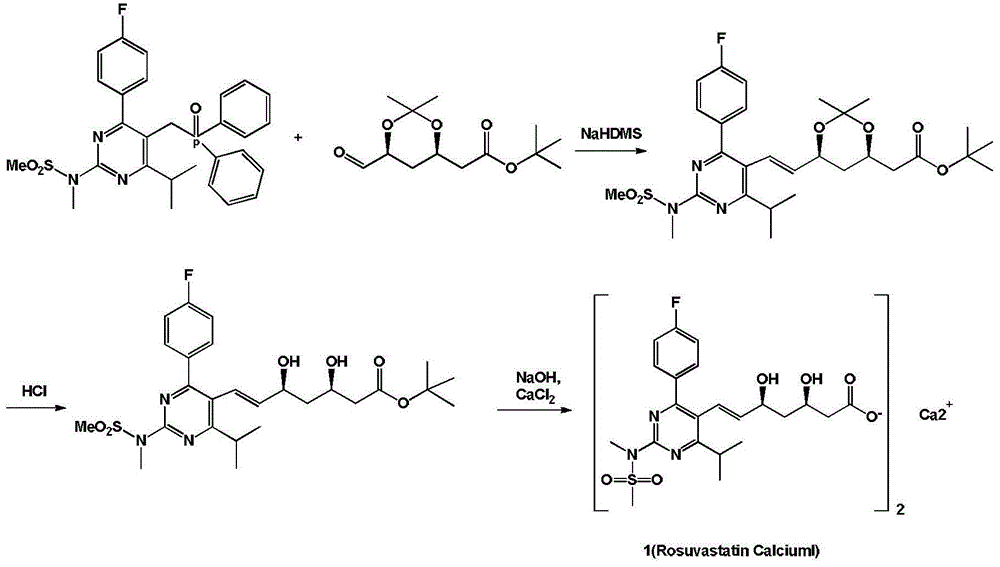
Rosuvastatin calcium preparation intermediate, and method for preparing rosuvastatin calcium from intermediate
A technology for rosuvastatin calcium and intermediates, which is applied in the field of preparation of intermediate compounds of rosuvastatin calcium and its preparation, can solve the problems of increasing the difficulty of controlling impurities in intermediates and finished products, and achieve the effect of reducing difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of Example 1 Compound 3-b (R=tert-butyl, X=Br)
[0053]
[0054] In a 100ml three-neck round bottom flask, compound 3-a 6.5g (22.5mmol), pyridine 11.2g (138mmol) were dissolved in 25ml of dichloromethane, under N 2Under protection, lower the temperature to -70°C to -80°C. Then, 4.2 g (13.5 mmol) of bis(trichloromethyl)carbonate was dissolved in 30 ml of dichloromethane, and this solution was added dropwise to the above reaction liquid at -70°C to -80°C. After the dropwise addition, the reaction solution was continuously stirred for 30 min to 60 min, and then the temperature of the reaction solution was slowly raised to room temperature, and then continued to stir for 1 h to 2 h. After the reaction of raw material 3-a was detected by TLC, after-treatment was carried out. Saturated ammonium chloride aqueous solution was added dropwise to the above reaction solution to terminate the reaction until no bubbles were generated. Then the reaction solution was qu...
Embodiment 2
[0055] Synthesis of Example 2 Compound 3 (R=tert-butyl, X=Br)
[0056]
[0057] In a 250ml three-neck round bottom flask, add DMF 50ml, compound 3-b 3.1g (10mmol), NaIO 4 2.1g (10mmol) and stirred to dissolve. The solution was heated to reflux (about 150°C) for 1h-2h. After the reaction of raw materials was monitored by TLC spot plate, post-processing was carried out. After the reaction solution was cooled to room temperature, 100 ml of water was added, and then 40 ml of ethyl acetate was used to extract 3 times. The ethyl acetate phases were combined, dried with anhydrous magnesium sulfate, filtered, and the ethyl acetate solvent was concentrated to obtain a light yellow solid. After recrystallization from petroleum ether / ethyl acetate, 2.1 g of compound 3 was obtained as a white solid, with a yield of 86.1%. 1 H NMR (400M, CDCl 3 )δ: 9.58(s, 1H), 4.50(m, 1H), 4.28(m, 1H), 2.68(m, 2H), 2.34(m, 2H), 1.45(s, 9H). MS (m / z): [M+H] + = 245.18.
Embodiment 3
[0058] Synthesis of Example 3 Compound 3-b (R=methyl, X=Cl)
[0059]
[0060] In a 100ml three-necked round bottom flask, 3.9g (20.0mmol) of compound 3-a, 10.0g (122.8mmol) of pyridine were dissolved in 23ml of dichloromethane, and the 2 Under protection, lower the temperature to -70°C to -80°C. Then, 3.7 g (12.0 mmol) of bis(trichloromethyl)carbonate was dissolved in 28 ml of dichloromethane, and this solution was added dropwise to the above reaction liquid at -70°C to -80°C. After the dropwise addition, the reaction solution was continuously stirred for 30 min to 60 min, and then the temperature of the reaction solution was slowly raised to room temperature, and then continued to stir for 1 h to 2 h. After the reaction of raw material 3-a was detected by TLC, after-treatment was carried out. Saturated ammonium chloride aqueous solution was added dropwise to the above reaction solution to terminate the reaction until no bubbles were generated. Then the reaction solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com