Method for detecting and analyzing multiple EDCs (endocrine disruptor chemicals) in environments and food
A technology of endocrine disruptors and analysis methods, which is applied in the direction of analysis materials, measuring devices, material separation, etc., can solve the problems of low concentration of endocrine disruptors, high derivatization reaction temperature, easy hydrolysis of derivatization reagents, etc., to achieve stable derivative products and improve Chromatographic resolution and detection sensitivity, the effect of improving method sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
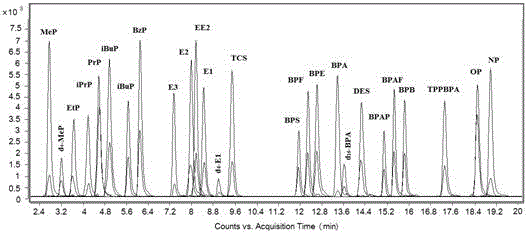
[0038] Chromatographic separation and qualitative and quantitative analysis of 23 endocrine disruptors and 3 internal standards:
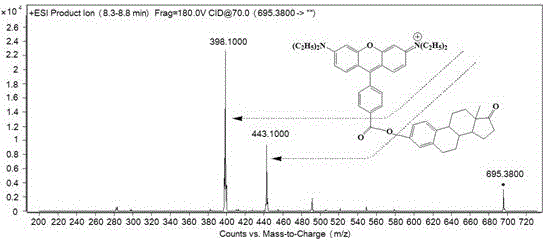
[0039] 23 kinds of endocrine disruptors and 3 kinds of internal standards (purchased from Sigma and Aladdin Reagent Company) were prepared in acetonitrile to obtain a concentration of 1.0 × 10 -5 mol / L standard solution of endocrine disruptors, take 0.2 mL from each single standard stock solution and dilute to 10 mL to obtain a mixed standard stock solution of endocrine disruptors. 4'-Carbonyl chloride-rhodamine was dissolved in acetonitrile to give 1.0 × 10 -2 mol / L derivatization reagent acetonitrile solution; take 70 µL of the standard solution and place it in a 1.5 mL centrifuge tube, add 30 µL of the internal standard solution (the internal standard solution is 10 µL with a concentration of 1×10 -5 mol / L estrone-2,4,16,16-d 4 , 10 µL concentration is 1×10 -5 mol / L Bisphenol A-d 16 , 10 µL concentration is 1×10 -5 mol / L methyl-4-hydro...
Embodiment 2
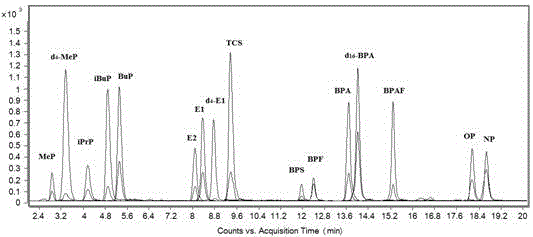
[0044] The detection and analysis of endocrine disruptors in domestic wastewater includes the following steps:
[0045] Take 1000 µL of domestic wastewater and filter it with a 0.45 µm water filter membrane, add HCl to adjust the pH to 6.0, then take 100 µL of water sample and place it in a 1.5 mL centrifuge tube, add 30 µL of internal standard solution (10 µL concentration is 1×10 -5 mol / L estrone-2,4,16,16-d 4 , 10 µL concentration is 1×10 -5 mol / L Bisphenol A-d 16 , 10 µL concentration is 1×10 -5 mol / L methyl-4-hydroxybenzoate-2,3,5,6-d 4 ), add 800 µL NaHCO 3 -Na 2 CO 3 (pH 11.5) buffer solution, and quickly inject 200 µL bromobenzene and 300 µL molar concentration of 1×10 -2 mol / L 4'-carbonyl chloride-rhodamine-acetonitrile solution, vortex mixer for 5 s to mix the solution evenly. After ultrasonic oscillation at 25 ºC for 1 min, centrifuge at high speed for 2 min at 12000 r / min. The organic phase was deposited at the bottom of the centrifuge tube, and the dep...
Embodiment 3
[0047] The detection of endocrine disruptors in river sediments includes the following steps:
[0048] references( Journal of Chromatography A , 2013, 1305: 17–26), take 0.8 g of river sediment samples and place them in a 20 mL centrifuge tube, add 3 mL of methanol, perform ultrasonic extraction for 30 min, centrifuge for 10 min, filter, and transfer the supernatant to a vial , as mentioned above, the residue was extracted again; the supernatant was combined and rotary evaporated to about 1 mL, and the volume was adjusted to 10 mL with ultrapure water, and 90 µL was placed in a 1.5 mL centrifuge tube, and 30 µL of the internal standard solution ( 10 µL concentration is 1×10 -5 mol / L estrone-2,4,16,16-d 4 , 10 µL concentration is 1×10 -5 mol / L Bisphenol A-d 16 , 10 µL concentration is 1×10 -5 mol / L methyl-4-hydroxybenzoate-2,3,5,6-d 4 ), add 900 µL of NaHCO pH 8.0 3 -Na 2 CO 3 buffer solution, and quickly injected with 120 µL 1-bromo-3-methylbutane and 200 µL molar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| detection limit | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com