Method for preparing hexa-fluorine lithium ferrite and carbon nano-tube composite materials
A technology of lithium hexafluoroferrate and carbon nanotubes, which can be used in electrical components, electrochemical generators, battery electrodes, etc., and can solve the problems of poor conductivity of lithium hexafluoroferrate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] Preparation method of lithium hexafluoroferrate and carbon nanotube composite material:
[0017] ①Dissolve 20 grams of ferric nitrate nonahydrate in 200 ml of deionized water, use 5 mg of cetyltrimethylammonium bromide as a surfactant, and keep stirring for 3 hours to form ferric nitrate-cetyltrimethylammonium bromide Saturated solution of methyl ammonium bromide;
[0018] ② Add 0.1 g of carbon nanotubes to 20 ml of 1 mol / L sodium hydroxide solution and stir for 3 hours, wash with deionized water until neutral, and centrifugally filter;
[0019] ③ Add the carbon nanotubes treated in step ② into 20 ml of 40% hydrofluoric acid solution, and continue stirring for 3 hours to obtain a relatively uniformly dispersed carbon nanotube-hydrofluoric acid solution;
[0020] ④Add 20 milliliters of carbon nanotube-hydrofluoric acid solution obtained in step ③ and 5.6 grams of lithium carbonate powder to 200 milliliters of ferric nitrate-hexadecyltrimethylammonium bromide solution ob...
Embodiment 1
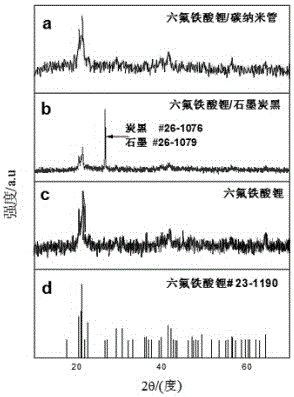
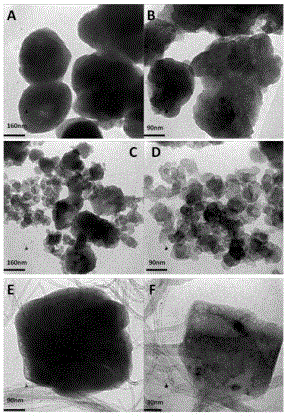
[0023] One: Comparative experiments of lithium hexafluoroferrate and carbon nanocomposite materials, lithium hexafluoroferrate and graphite carbon black nanocomposites, and lithium hexafluoroferrate:
[0024] 1. The preparation method of lithium hexafluoroferrate:
[0025] 1) Dissolve 20 grams of ferric nitrate nonahydrate in deionized water, use 5 mg of cetyltrimethylammonium bromide as a surfactant, and continue stirring for 3 hours to form ferric nitrate-cetyltrimethylammonium ammonium bromide saturated solution;
[0026] 2) Add 20 ml of 40% hydrofluoric acid solution and 5.6 g of lithium carbonate powder to the ferric nitrate-hexadecyltrimethylammonium bromide saturated solution obtained in step 1), and continue stirring for 3 hours. white precipitate;
[0027] 3) Wash the white precipitate obtained in step 2) with isopropanol for 5 times, and then put it in an air-blast drying oven at 80°C for 10 hours to obtain a lithium hexafluoroferrate sample.
[0028] 2. Preparati...
Embodiment 2
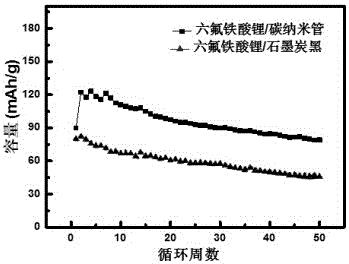
[0033] 1: Lithium hexafluoroferrite and carbon nanotube composite material and lithium hexafluoroferrate and graphite carbon black nanocomposite for electrode comparison experiment:
[0034] 1. Mix 0.8 grams of lithium hexafluoroferrate with graphite carbon black nanocomposites and 0.2 grams of polyvinylidene fluoride, then add 8 milliliters of N-methyl-2-pyrrolidone, and ball mill for 1 hour to obtain experimental Slurry of lithium hexafluoroferrate and graphite carbon black nanocomposite.
[0035] 2. Mix 0.8 g of lithium hexafluoroferrate with carbon nanotube composite material and 0.2 g of polyvinylidene fluoride, then add 8 ml of N-methyl-2-pyrrolidone, and ball mill for 1 hour to obtain the experimental Slurry of lithium hexafluoroferrate / carbon nanotube composite.
[0036] 3. Use aluminum foil as the current collector; first punch the aluminum foil into a small disc with a diameter of 1 cm and a piece with a width of 1.5 × 5 cm. After removing the burr, clean it with ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com