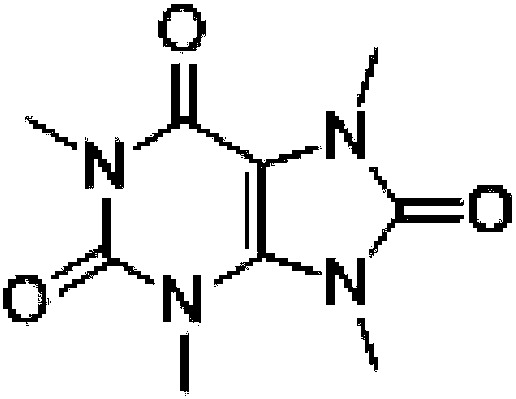
A kind of total synthesis method of 1,3,7,9-tetramethyluric acid
A technology of tetramethyl uric acid and dimethyl uric acid, applied in the direction of organic chemistry, etc., can solve the problems of high equipment requirements, cumbersome separation, high risk, etc., and achieves the effects of low equipment requirements, simple post-processing, and high conversion rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
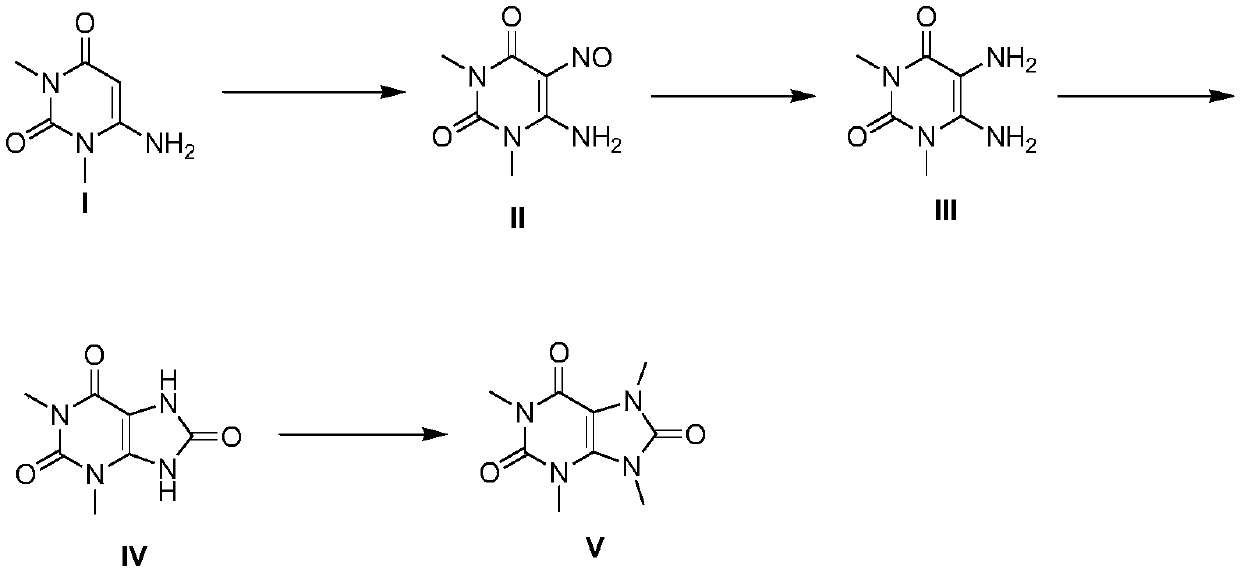
[0051] A kind of total synthesis method of 1,3,7,9-tetramethyluric acid, comprises the steps:
[0052] S1: Nitrosylation reaction: use 6-amino-1,3-dimethyluracil as the raw material, as compound I, weigh compound I and dissolve it in ethanol, the mass / volume of compound I and ethanol is 1:8 ~1:20;
[0053] Slowly add the nitrosation reagent dropwise, wherein the mass ratio of compound I to the nitrosation reagent is 3:1 to 3:5, stir at room temperature after the dropwise addition, and monitor the reaction process by HPLC. After the reaction is completed, cool down , filtered, and vacuum-dried to obtain compound II;
[0054] S2: Reduction reaction: Weigh compound II and dissolve it in methanol, the mass / volume of compound II and methanol is 1:3-1:8; add insurance according to the mass ratio of compound II and sodium bicarbonate at 5:3-5:7 powder, fully reacted at room temperature for 12-20h, monitored by HPLC, concentrated after the reaction;
[0055] Add distilled water to ...
Embodiment 2
[0065] A kind of total synthesis method of 1,3,7,9-tetramethyluric acid, comprises the steps:
[0066] S1: Preparation of compound Ⅱ
[0067] ① Weigh 10kg of 6-amino-1,3-dimethyluracil and dissolve it in 120L of ethanol to obtain solution A;
[0068] ② Weigh 7.82kg of isoamyl nitrite, slowly add isoamyl nitrite dropwise to solution A, stir at room temperature after the dropwise addition, and leave overnight;
[0069] ③ HPLC monitoring, after the reaction was completed, the temperature was lowered, filtered, and vacuum-dried to obtain 10.5 kg of red solid, namely compound II, with a yield of 90.0%.
[0070] H NMR spectrum data of compound Ⅱ: 1 H-NMR (CDCl 3 ,400MHz): 2.72(s,6H), 2.12(br,2H).
[0071] S2: Preparation of compound Ⅲ
[0072] ① Weigh 10kg of compound II and dissolve it in 50L of methanol, add 14.1kg of sodium hydrosulfite, fully react at room temperature for 12-20h, monitor by HPLC, and concentrate after the reaction is completed;
[0073] ② Add 20L of distil...
Embodiment 3
[0093] S1: Preparation of compound Ⅱ
[0094] ① Weigh 10kg 6-amino-1,3-dimethyluracil and dissolve it in 100L ethyl acetate to obtain solution A;
[0095] ② Weigh 9.53kg of butyl nitrite, slowly add butyl nitrite dropwise to solution A, stir at room temperature after the dropwise addition, and leave overnight;
[0096] ③ HPLC monitoring, after the reaction was completed, the temperature was lowered, filtered, and vacuum-dried to obtain 10.2 kg of a red solid, which was compound II.
[0097] S2: Preparation of compound Ⅲ
[0098] ①Weigh 10kg of compound II and dissolve it in 80L dimethyl sulfoxide, add 16kg of hydrosulfite, fully react at room temperature for 12-20h, monitor by HPLC, and concentrate after the reaction is completed;
[0099] ② Add 20L of distilled water to the above reactant to dissolve, add 20L of DCM to extract, recover the organic phase, repeat the extraction three times, and combine the organic phase;
[0100] ③ Add 10 L of saturated brine to the above or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com