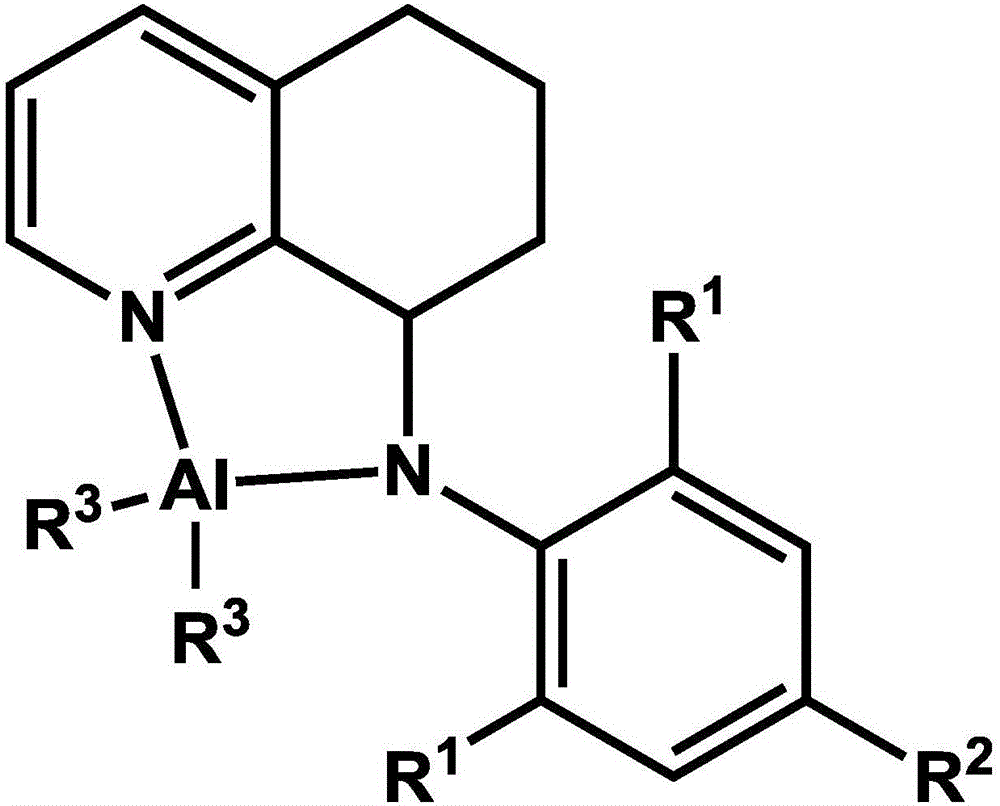
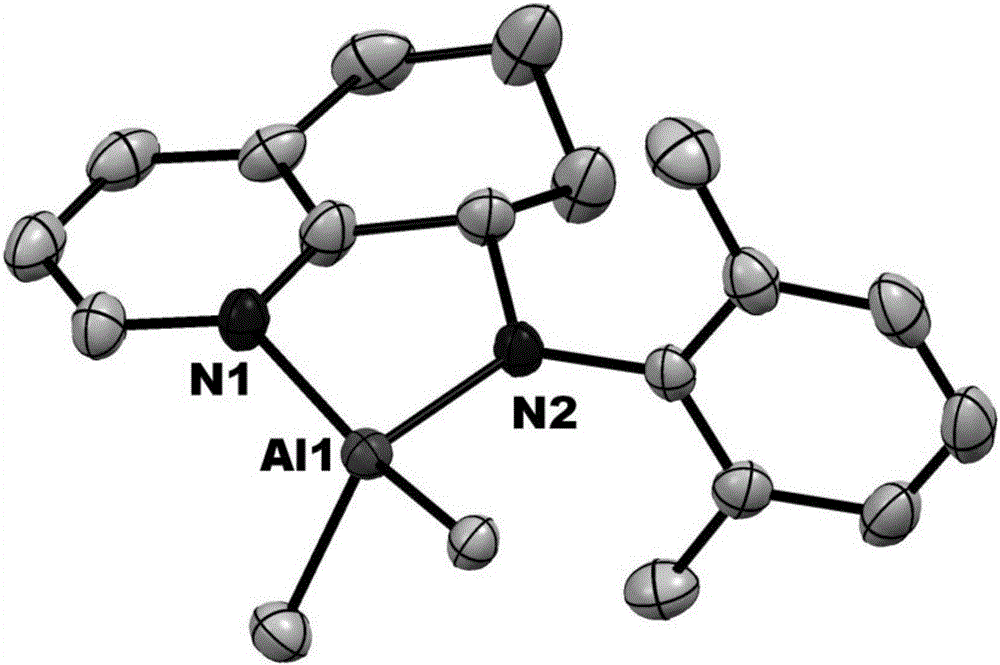
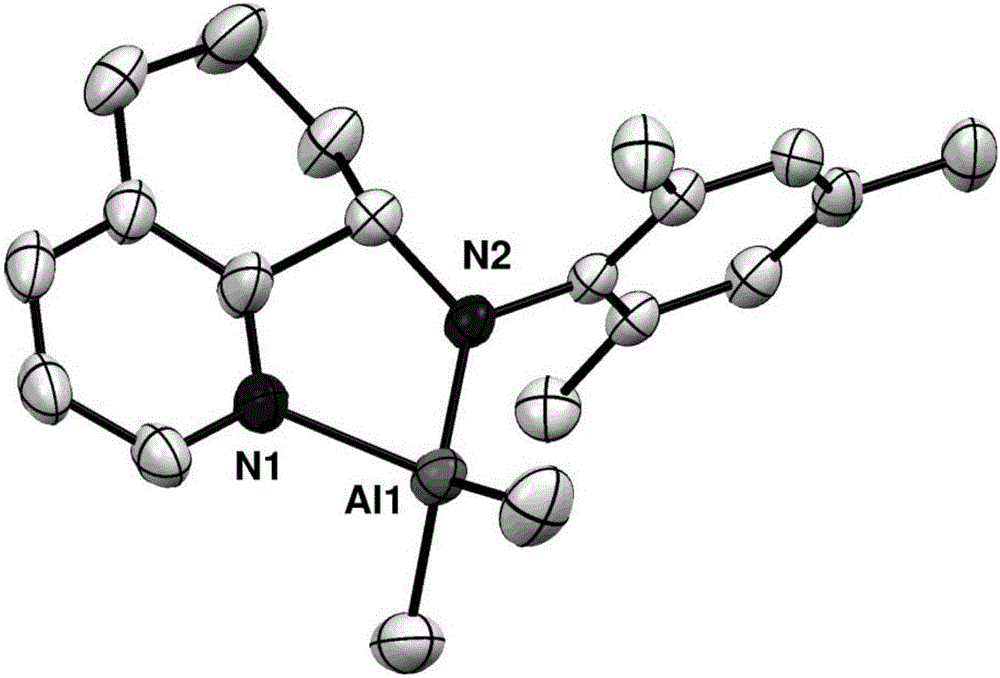
8-N-arylamine-hydrogenation quinoline complexation aluminum alkyl compound and preparation method and application thereof
A kind of hydrogenated quinoline, alkyl aluminum technology, applied to the application of lactone and lactide ring-opening polymerization, 8-N-arylamine-hydrogenated quinoline complex alkyl aluminum compound and its preparation field, to achieve product yield The effect of high rate, stable properties, and easy control of catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, preparation of ligand L1 (8-N-2,6-diisopropylaniline-hydroquinoline)
[0048] Under nitrogen atmosphere, 5,6,7-trihydroquinolin-8-one (1.48g, 10mmol), 2,6-diisopropylaniline (1.95g, 11mmol) and toluenesulfonic acid (20mg ) was dissolved in 50mL toluene, and refluxed for 12 hours. The toluene solvent was removed under reduced pressure, 50 mL of methanol and 50 mL of dichloromethane were added to dissolve, 6 equivalents of sodium borohydride (2.28 g, 60 mmol) was slowly added, and the reaction was stirred at room temperature for 10 hours. Silica column chromatography separated to obtain a yellow solid L11.31g, 5.60mmol, yield 56.0%.
[0049] FT-IR (KBr, cm -1 ):3311, 3060, 2955, 2864, 1574, 1457, 1417, 1324, 1250, 1194, 1147, 1104, 1055, 1002, 935, 807, 781, 745, 707, 546. 1 HNMR (CDCl 3 ): δ8.48(d, 1H, J=4.5Hz, quino-H), 7.42(d, 1H, J=7.6Hz, quino-H), 7.14-7.11(m, 4H, quino-H+Ar- H), 4.46 (br, 1H, N-H), 4.04 (dd, 1H, J=8.5, 4.6Hz, NCH), 3.59 (hept, 2H,...
Embodiment 2
[0050] Example 2, preparation of ligand L2 (8-N-2,6-diethylaniline-hydroquinoline)
[0051] The experimental procedure is the same as that in Example 1, the yield of ligand L2 (8-N-2,6-diethylaniline-hydroquinoline): 1.73 g, 6.20 mmol, 62.0% yield.
[0052] FT-IR (KBr, cm -1 ):3341, 3043, 2958, 2904, 1564, 1460, 1415, 1325, 1240, 1194, 1176, 1123, 1056, 938, 827, 780, 746, 717, 542. 1 HNMR (CDCl 3 ): δ8.48(d, 1H, J=4.2Hz, quino-H), 7.43(d, 1H, J=7.6Hz, quino-H), 7.16-7.12(m, 4H, quino-H+Ar- H), 4.38(br, 1H, N-H), 4.10(dd, 1H, J=8.4, 4.3Hz, NCH), 2.89-2.78(m, 2H, quino-H), 2.35(q, 4H, J=6.9 Hz, CH 2 CH 3 ), 2.09-1.97(m, 2H, quino-H), 1.80-1.65(m, 2H, quino-H), 1.29(t, 6H, J=6.9Hz, CH 2 CH 3 ). 13 CNMR (CDCl 3 ): δ157.42, 148.11, 146.62, 141.98, 135.91, 132.15, 125.06, 123.57, 122.98, 60.55, 29.77, 28.80, 27.89, 24.22, 20.20.Anal.CalcdforC 21 h 28 N 2 : C, 81.38; H, 8.63; N, 9.99. Found: C, 81.35; H, 8.52; N, 9.72.
Embodiment 3
[0053] Example 3, preparation of ligand L3 (8-N-2,6-dimethylaniline-hydroquinoline)
[0054] The experimental procedure is the same as that in Example 1, and the yield of ligand L3 (8-N-2,6-dimethylaniline-hydroquinoline): 1.28 g, 5.08 mmol, 50.8%.
[0055] FT-IR (KBr, cm -1 ):3333, 3042, 2940, 1589, 1570, 1471, 1438, 1256, 1214, 1186, 1160, 1093, 1033, 1013, 877, 846, 791, 752, 705, 681, 571. 1 HNMR (CDCl 3 ): δ8.47 (d, 1H, J = 4.3Hz, quino-H), 7.42 (d, 1H, J = 7.6Hz, quino-H), 7.12 (dd, 1H, J = 7.6, 4.7Hz, quino -H), 7.02(d, 2H, J=7.4Hz, Ar-H), 6.87(t, 1H, J=7.4Hz, Ar-H), 4.37-4.36(m, 1H, NCH), 4.01(br , 1H, NH), 2.94-2.72(m, 2H, quino-H), 2.33(s, 6H, Me), 2.03-1.86(m, 2H, quino-H), 1.85-1.71(m, 2H, quino-H) -H). 13 CNMR (CDCl3): δ157.75, 147.32, 145.01, 136.88, 132.07, 131.28, 128.80, 122.21, 122.06, 57.32, 29.81, 28.70, 19.61, 19.09. 17 h 20 N 2 : C, 80.91; H, 7.99; N, 11.10. Found: C, 80.78; H, 8.15; N, 11.02.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com