Parainfluenza virus type-1, 2, 3 chemiluminescence immunoassay kit and preparation method thereof
A technology of chemiluminescent immunity and detection kits, applied in chemiluminescence/bioluminescence, analysis by making materials react chemically, measuring devices, etc., can solve cross-contamination of reagents, cannot perform single-serve detection and filling Reagent operation is cumbersome and other problems, to achieve the effect of high detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
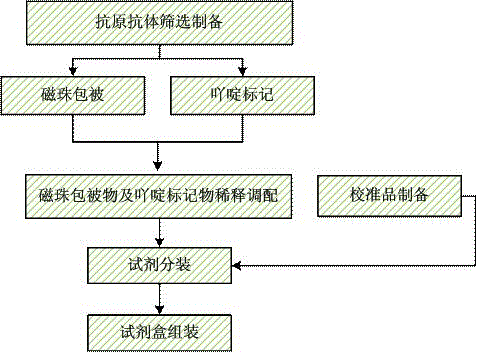
[0055] Such as figure 1 The preparation method of the above-mentioned parainfluenza virus type 1, 2, and 3 chemiluminescence immunoassay kits shown comprises the following steps:
[0056] Take the suspension of carboxylated magnetic particles, remove the supernatant by magnetic separation, resuspend with MES buffer, then add EDC aqueous solution to activate the carboxyl groups on the surface of carboxylated magnetic particles, and then add parainfluenza virus type 1, 2, and 3 for recombination The protein was suspended at room temperature for 2h-10h, and the supernatant was removed by magnetic separation, and then resuspended with Tris buffer to obtain carboxylated magnetic particles coated with parainfluenza virus type 1, 2, and 3 recombinant proteins.
[0057] MES (2-(N-morpholine)ethanesulfonic acid) buffer had a concentration of 0.02M and a pH of 5.5.
[0058] Tris buffer has a concentration of 0.1 M and contains 2% BSA, pH 8.0.
[0059] The concentration of EDC (1-ethyl...
Embodiment 1
[0075] Example 1: Preparation of parainfluenza virus type 1, 2, and 3 chemiluminescent immunoassay kits
[0076] (1) Preparation of carboxylated magnetic particles coated with parainfluenza virus type 1, 2, and 3 recombinant proteins:
[0077] Take the suspension containing 50 mg of carboxylated magnetic particles (MagnaBind21353) with a particle size of 0.05 μm~1 μm, magnetically separate to remove the supernatant, resuspend with 0.02 M, pH 5.5 MES buffer, add 1 mL of newly prepared 10 mg / mL EDC aqueous solution, activate the carboxyl groups on the surface of magnetic beads, add 4 mg of parainfluenza virus type 1, 2, and 3 recombinant proteins (biorbyt, product number orb48780), suspend at room temperature for 6 hours, magnetically separate, remove the supernatant, and wash with 0.1M containing 2% BSA , resuspended to 1 mg / mL in Tris buffer with a pH of 8.0 to obtain carboxylated magnetic particles coated with recombinant proteins of parainfluenza virus types 1, 2, and 3, and...
Embodiment 2
[0082] Embodiment 2: Parainfluenza virus type 1, 2, 3 chemiluminescent immunoassay method
[0083] The automatic chemiluminescent immunoassay analyzer (YHLO, catalog number iFlash3000) was used as the detection tool, and the methodological mode was indirect immunoassay, that is, 50 μL of samples and 50 μL of parainfluenza virus type 1, 2, and 3 recombinant protein packages were sequentially added to the instrument. Carboxylated magnetic particles and 50 μL of parainfluenza virus type 1, 2, and 3 treatment solutions were reacted for 20 minutes, and then 50 μL of anti-human immunoglobulin acridinium ester was added for 20 minutes of reaction before magnetic separation. , the instrument sends the reaction mixture into the dark room, and sequentially adds the luminescent substrate A solution (H 2 o 2 ) and solution B (NaOH) for luminescence reaction, and finally record the luminescence value.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com