Compositions and methods for modifying the surface of cells and methods of use
A composition and cell technology, applied in the described field, can solve the problems of cytotoxicity and damage to the normal function of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091] a. Compound preparation
[0092] Certain compounds described herein may be commercially available. Others may require synthetic preparation. For the preparation of compounds of formula I, a phospholipid-PEG intermediate bearing a reactive functional group on the terminus of the PEG can be coupled to a targeting moiety.
[0093] For example, when the reactive functional group is NHS, the intermediate can be coupled to a targeting moiety with a reactive amino group to form an amide. When the reactive functional group is an amine, the intermediate can be coupled with a reactive isothiocyanate to form a thiocarbamate. When the reactive functional group is azide, the intermediate can be coupled with a targeting moiety with a reactive nitrile or reactive alkyne group to form a tetrazole or triazole. When the reactive functional group is maleimide, the intermediate can be coupled to a targeting moiety with a reactive thiol group to form a bond between the sulfur of the thio...
Embodiment 1
[0122] compound synthesis
[0123] General experimental and analytical details
[0124] Functionalized DMPE-PEG (3.4 kDa and 5 kDa) were purchased from Nanocs Corporation (New York, NY). Fluorescein isothiocyanate (FITC) and rhodamine were purchased from ThermoScientific (Rockford, IL).
[0125] in CDCl with 0.03% TMS as internal standard 3 Medium or DMSO-d 6 Recorded on a Bruker AM 400 spectrometer (operated at 400 and 101 MHz, respectively) or a Bruker AVIII spectrometer (operated at 500 and 126 MHz, respectively) 1 H and 13 C NMR spectra. Chemical shifts (δ) are in parts per million (ppm) and coupling constants (J) are in Hertz (Hz). Spin multiplicity is reported as s = singlet, br.s = broad singlet, d = doublet, t = triplet, q = quartet, dd = double doublet, and m = multiplet. LCMS analysis was performed on an Agilent 1200 RRL chromatograph with photodiode array UV detection and an Agilent 6224 TOF mass spectrometer. Chromatography used the following parameters: Wa...
Embodiment 2
[0139] MSC modification
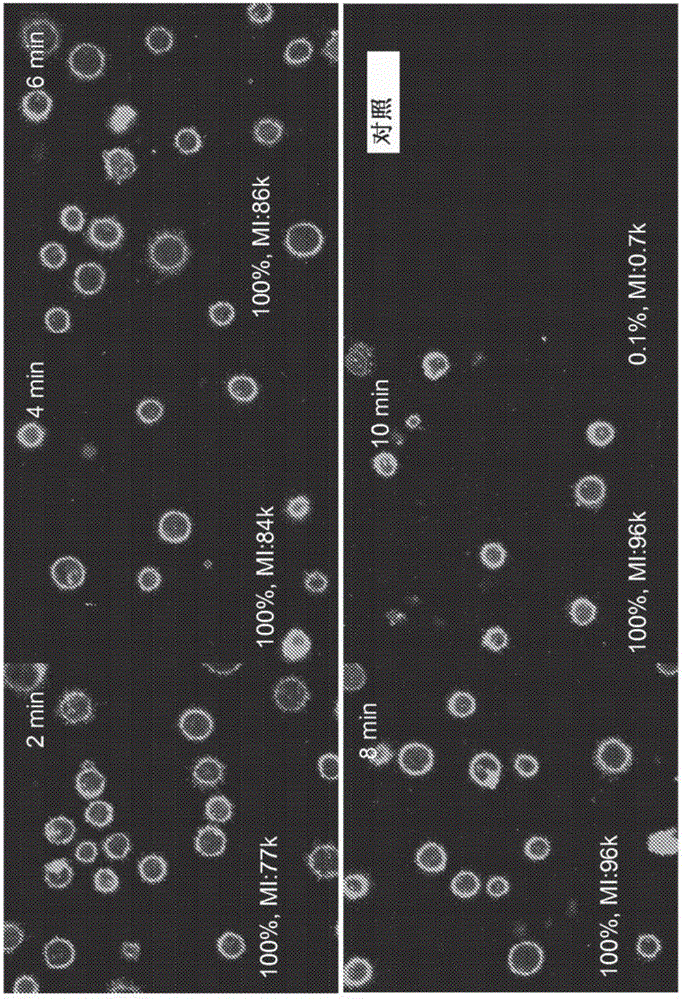
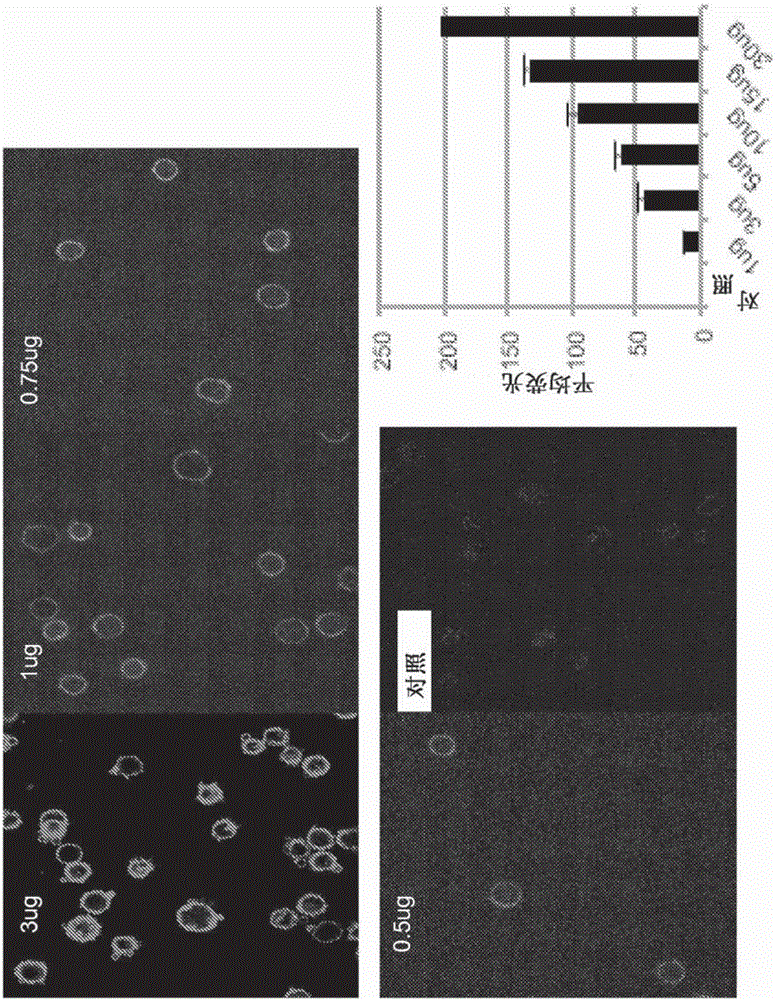
[0140] General Experimental Procedure : Culture-expanded MSCs were harvested and suspended in phenol red-free medium. DMPE-PEG or DMPE-PEG-CXCR4 was added directly to the cell suspension. At predetermined time points, MSCs were harvested, washed, and suspended in HBSS. To optimize the amount of DMPE-PEG, different amounts of DMPE-PEG were mixed with 750,000 MSCs.
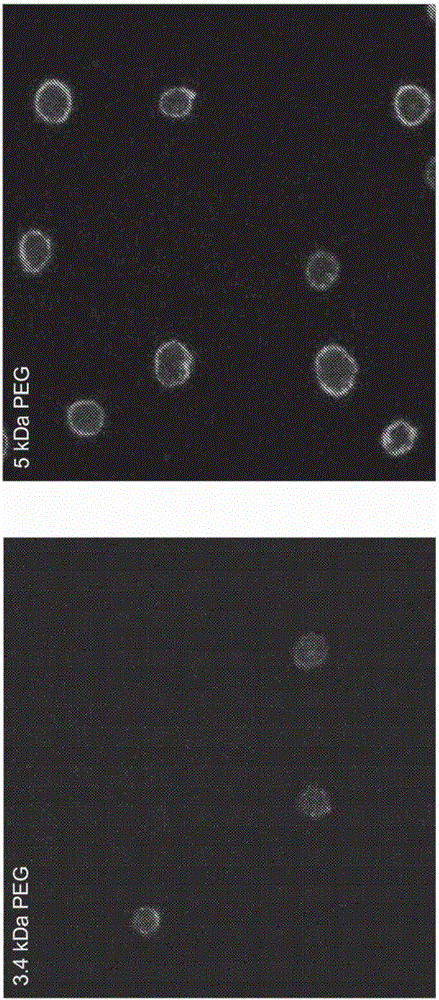
[0141] Effect of PEG size on MSC surface modification : Two DMPE-PEG compositions with different PEG sizes, namely 5kDa and 3.4kDa, were labeled with FITC for fluorescence detection, and the DMPE-PEG-FITC compounds were subsequently incubated with MSCs in suspension. The ability of compounds to bind to the surface of MSCs was assessed by confocal microscopy and FACS analysis (see figure 1 ). The results show that the incorporation of 5kDa PEG into the composition is superior to the use of 3.4kDa PEG. All subsequent experiments described herein used DMPE-PEG compositions with 5 kDa ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com