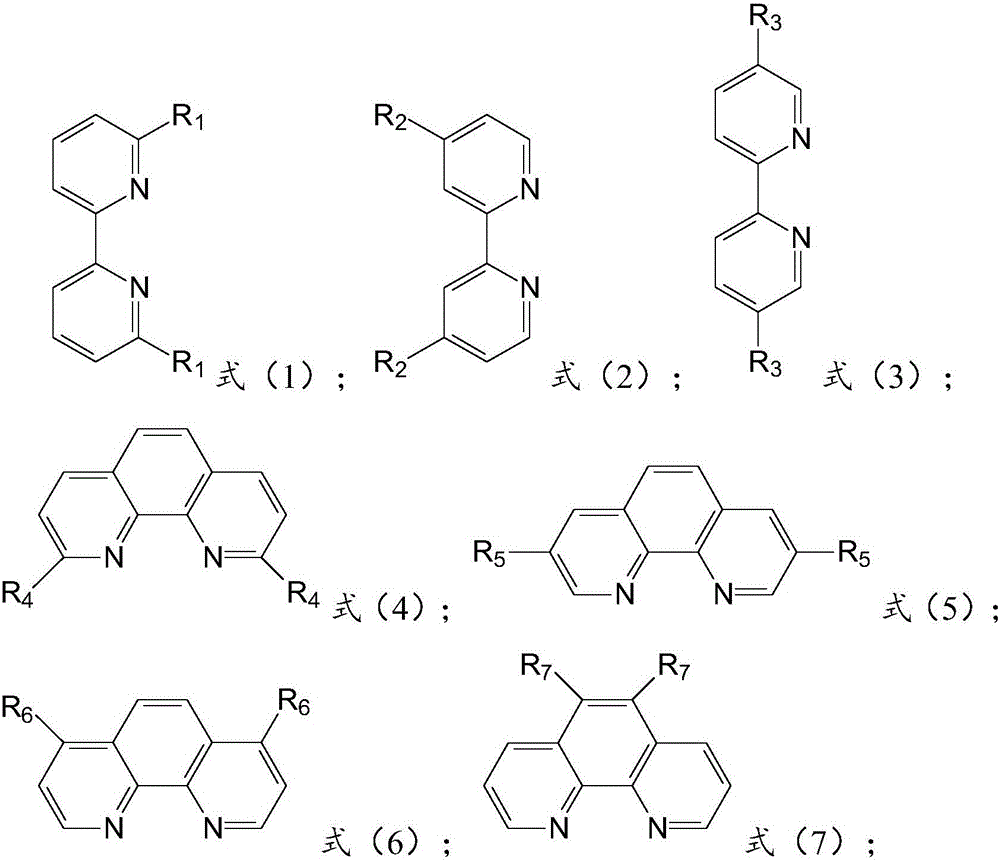
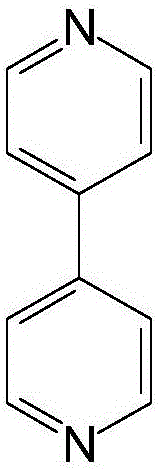
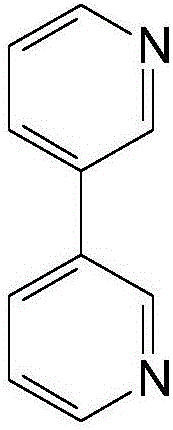
Method for preparing 2,2'-dimethylbiphenyl
A technology of dimethyl biphenyl and o-chlorotoluene, applied in chemical instruments and methods, hydrocarbon production from halogen-containing organic compounds, catalysts, etc., can solve the problems of long reaction time, large amount of solvent THF, long time, etc. Improve conversion rate and selectivity, meet industrial production needs, improve conversion rate and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 5 parts catalyst NiCl 2 (TPP) 2 , 100 parts of o-chlorotoluene, 100 parts of tetrahydrofuran, 20 parts of magnesium powder, 0.5 part of nitrogen-containing co-ligand 4,4-bipyridine and 0.1 part of initiator iodine were added to the three-necked flask, stirred and heated until the reaction started, and the A mixture of 100 parts of o-chlorotoluene and 100 parts of tetrahydrofuran (THF) was added dropwise into the dropping funnel, heated to reflux for 5 hours, the reaction was stopped, and THF was recovered by distillation. Add an aqueous solution containing 10-15wt% HCl, separate the liquids, wash the organic layer twice with water, dry over magnesium sulfate, filter, and distill under reduced pressure to collect 2,2′-dimethylbiphenyl fractions, and analyze the conversion rate of raw materials by gas chromatography The selectivity to 2,2'-dimethylbiphenyl was 95%, and the selectivity to 2,2'-dimethylbiphenyl was 86.0%.
Embodiment 2
[0044] 2 parts catalyst NiCl 2 (TPP) 2 , 100 parts of o-chlorotoluene, 100 parts of tetrahydrofuran, 20 parts of magnesium powder, 0.5 parts of nitrogen-containing co-ligand 2,2-bipyridine and 0.1 part of initiator iodine were added to the three-necked flask, stirred and heated until the reaction started, and the Add a mixture of 100 parts of o-chlorotoluene and 100 parts of tetrahydrofuran (THF) dropwise into the dropping funnel, heat and reflux for 4 hours, stop the reaction, recover THF by distillation, add an aqueous solution containing 10 to 15 wt% of HCl, separate the liquid, and the organic layer Wash twice with water, dry over magnesium sulfate, filter, and distill under reduced pressure to collect 2,2′-dimethylbiphenyl fractions. The fractions are analyzed by gas chromatography and the conversion rate of raw materials is 90%, producing 2,2′-dimethylbiphenyl The selectivity was 82.0%.
Embodiment 3
[0046] 6 parts of catalyst NiCl 2 (TPP) 2 , 100 parts of o-chlorotoluene, 100 parts of tetrahydrofuran, 20 parts of magnesium powder, 0.5 parts of nitrogen-containing co-ligand 1,10-phenanthroline and 0.1 part of initiator iodine were added into a three-necked flask, stirred and heated until the reaction started, and the A constant pressure dropping funnel was added dropwise a mixed solution of 100 parts of o-chlorotoluene and 100 parts of tetrahydrofuran (THF), heated and refluxed for 2 hours, stopped the reaction, recovered THF by distillation, added an aqueous solution containing 10 to 15 wt% of HCl, and separated the liquids. The organic layer was washed twice with water, dried over magnesium sulfate, filtered, and the 2,2′-dimethylbiphenyl fraction was collected by distillation under reduced pressure. The conversion rate of the raw material of the fraction was 98.5% according to gas chromatography analysis, and 2,2′-dimethylbiphenyl was generated. The selectivity of biph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com