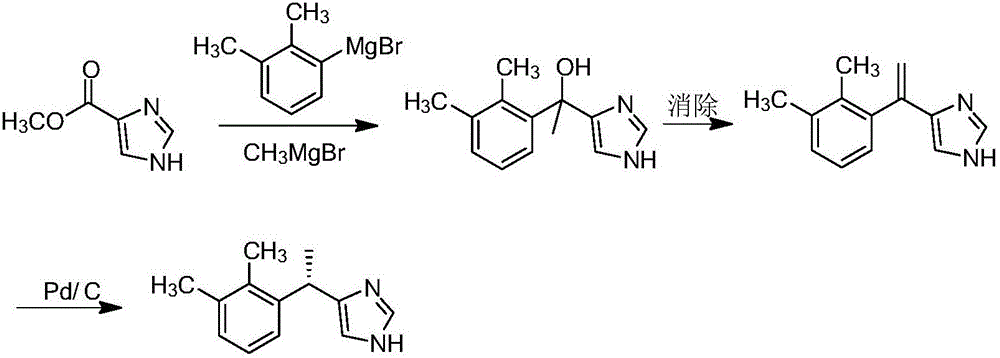
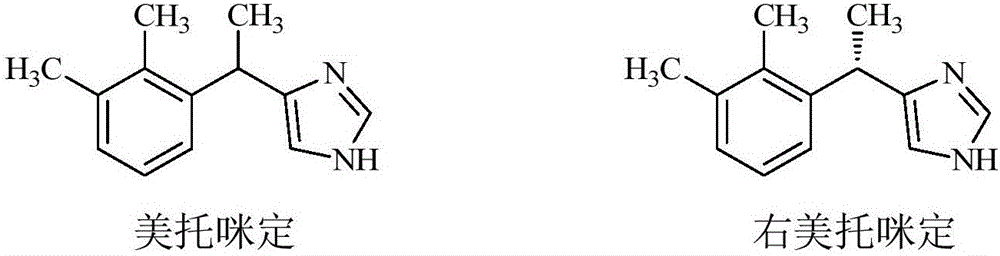Method for preparing dexmedetomidine
A technology for medetomidine and imidazole, which is applied in the field of preparation of dexmedetomidine hydrochloride and its intermediates, can solve the problems of reduced yield of main product and unscheduled reaction route, and achieve convenient operation and short synthetic route , to ensure high-quality results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
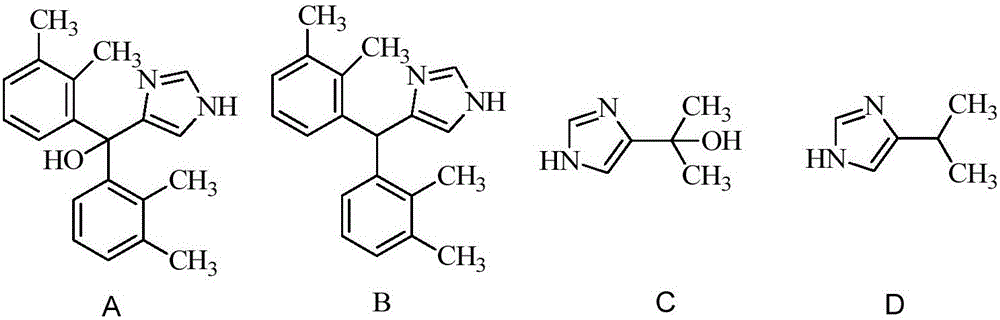
[0046] Example 1: Preparation of 1-(2,3-dimethylphenyl)-1-(1-trityl-1H-imidazol-4-yl)ethanol
[0047]
[0048] Step 1: Preparation of 1-trityl-1-H imidazole Grignard reagent
[0049] Under the protection of nitrogen, in a 1L reaction flask, add 4-iodo-1-trityl-1-H imidazole (144g, 330mmol) dissolved in 720ml of tetrahydrofuran, drop ethylmagnesium bromide (330mmol, 1M tetrahydrofuran solution), stirred at room temperature for 30 min, and set aside.
[0050] Step 2: preparation of formula I compound
[0051] Nitrogen protection, add anhydrous ZnCl in Schlenk reactor 2 (4.1g, 30mmol), LiCl (14g, 330mmol), tetrabutylammonium bromide (1M ether solution, 60mL, 60mmol), stirred at room temperature for 15 minutes. Add the tetrahydrofuran solution of (1-trityl-1H-imidazol-4-yl)magnesium bromide prepared above, stir at room temperature for 30 min, concentrate part of the solvent under reduced pressure to about 330 ml, and cool the mixture to 0 °C. Add a solution of 2,3-dimethyla...
Embodiment 13
[0068] Embodiment 13: the preparation of medetomidine
[0069]
[0070] In a 3L reaction flask, put 4-[(2,3-dimethylphenyl)-1-hydroxyethyl]-1-(triphenylmethyl)imidazole 46g (100mmol), triethylsilane 59.3 g, 1400ml of dichloromethane, cooled to -10°C, 80ml of trifluoroacetic acid was added dropwise under stirring, the dropwise was completed in about 1 hour, and the reaction was continued for 4 hours, then slowly raised to room temperature, and reacted overnight. Wash three times with 300ml of saturated sodium bicarbonate solution, once with 300ml of water, dry over anhydrous sodium sulfate, concentrate to dryness, add 650ml of ethyl acetate, extract with 2N hydrochloric acid (75ml×4), combine the extracts, add 10% Pd / C 0.7 g, normal pressure through H 2 Reduce overnight, filter with celite, neutralize with 20% sodium hydroxide solution, extract with ethyl acetate (300ml×2), combine the organic layers, wash with brine (150ml), dry over anhydrous sodium sulfate, and concentr...
Embodiment 14
[0072] Example 14: Preparation of (S)-dexmedetomidine-L-(+)-tartrate
[0073] L-(+)-tartaric acid (9 g, 60 mmol) was added to a solution of medetomidine (12 g, 60 mmol) in ethanol (250 ml). The suspension was heated to reflux until completely dissolved, then stirred overnight at room temperature, and filtered to obtain a white solid (9 g). The resulting solid was dissolved in isopropanol (200ml) under reflux, stirred overnight at room temperature, and filtered to obtain (13.5g). The obtained solid was refined again by the same method to obtain 8.1 g of solid with a purity of 99.9% and a yield of 77.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com